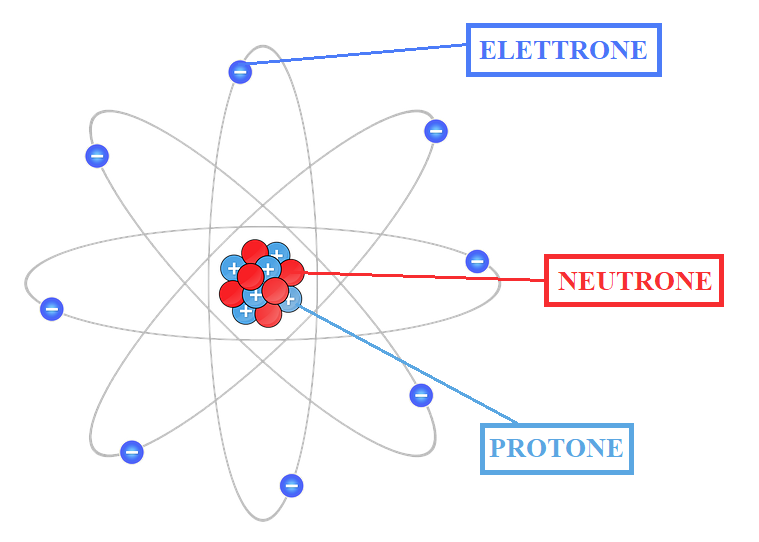
What are atoms?
In this article, we are going to learn what atoms are and how they are made. However, let’s start answering the question: what does the word atom mean?
The word atom, from Greek. átomos, means indivisible
since it was thought to be the smallest part of the matter, and poor Dalton believed so too.
The particles that make up the atom
Today, however, we know that the atom is itself made up of smaller particles. Indeed, it consists of a small and heavy nucleus of neutrons and protons, and of a cloud of light electrons that move around. The nucleus is hence very small. It occupies only a millionth of a billionth of the entire volume of the atom. Moreover, since nucleus holds most of the mass of the entire atom, this entails that it is very dense (the mass of the nucleus is 1800 times greater than that of an electron), while the remaining volume of the atom is made up mostly of empty space (see Figure 1).
Comparing an atom to a room, the nucleus would be a crumb, while the electrons would move along the walls.
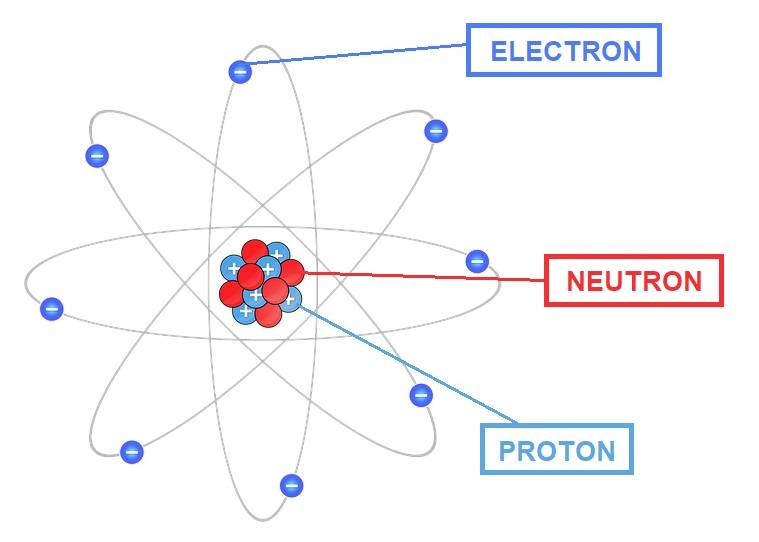
Protons and electrons are charged particles, whose charge is equal but of opposite sign (1.602×10-23 C). Indeed, protons have a positive charge, while electrons have a negative charge. Neutrons, on the other hand, are neutral particles but with a mass comparable to that of protons (1.67 × 10-24 g); the mass of the electrons is instead 9.11×10-28g.
Atoms: symbols and numbers
In order to distinguish the various types of atoms, chemical symbols are used. These symbols consist of one or two letters, which often come from the initial of the Latin or Greek name of the atom in question. For example, the word hydrogen derives from the Greek hýdor, “water”, and ghennáo, “generate”, i.e. “generator of water”. Hence, the chemical symbol for the hydrogen atom is H, from the initial of the Greek word. In the same way, the helium atom derives from the Greek word hélios, “sun”. Therefore, the chemical symbol of helium is the initials of the Greek word: He. And so on for the other elements.
To know the chemical symbols of atoms, just look at the Periodic Table where they are listed and you can memorize them!
Looking at the Periodic Table, however, you will also notice that the atoms, besides being distinguished by the chemical symbols, are also characterized by numbers. In fact, the correct way to represent an atom is to write the chemical symbol with two numbers, one at the top and the other at the bottom (Figure 2).

These two numbers are respectively the mass number (A, superscript) and the atomic number (Z, subscript). However, what are these two numbers?
- The atomic number (Z) is the number of protons, according to which atoms are classified within the periodic table; indeed, atoms with the same atomic number belong to the same element.
- The mass number (A), on the other hand, is the total number of neutrons and protons.
Therefore, the atomic number is very important because it identifies that specific type of atom. It is called atomic number, precisely because it is an identification number of that atom, a bit like a serial number identifies an item. The mass number, on the other hand, only gives us additional information relative to the total number of neutrons and protons and is so called precisely because it is the totality of protons and neutrons that gives the bulk of the mass to the atom, since electrons weigh much less.
Ions and isotopes
Atomic number and mass number are also very useful to identify and define ions and isotopes.
Ions
Let’s start talking about ions.
In a neutral atom, the number of protons is equal to that of electrons, since the charge of the electron is equal but with opposite sign to that of the proton. Therefore, in a neutral atom the number Z, besides indicating the number of protons, indicates the number of electrons too.

However, atoms can either lose electrons or gain them. In such a case, Z will only indicate the number of protons, because now the number of electrons has changed. In this case, we no longer speak of an atom but of an ion. Indeed:
- Atoms with different numbers of electrons and protons acquire a charge and are called ions
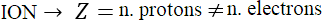
An ion that has obtained electrons is called ANION and acquires a negative charge. Conversely, an ion that loses electrons is called CATION and acquires a positive charge.
Let’s take an example, if hydrogen loses its only one electron it will become positively charged and will be indicated with the symbol: H+. In such a cation, Z is always equal to 1, but this time the number of electrons is equal to 0 (Figure 3).

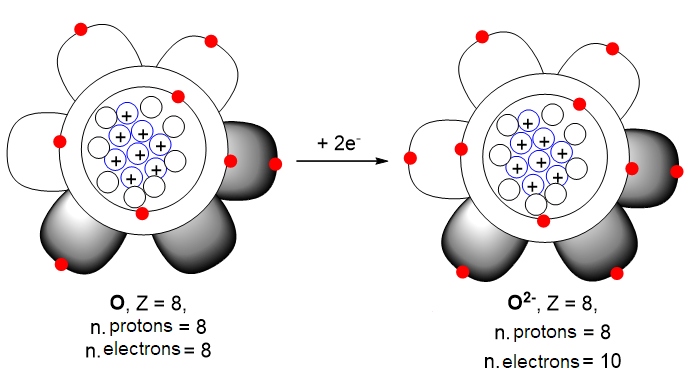
Similarly, if oxygen acquires two electrons it will become twice negatively charged and will be denoted by the symbol: O2-. In this anion, Z is always equal to 8, but the number of electrons is no longer 8, but 10, due to the two more electrons acquired by the atom (Figure 4).
Isotopes
Let us now understand what isotopes are
There are atoms with the same number of protons (same Z) but a different number of neutrons (different A), i.e. atoms that belong to the same element, but have different masses due to a lower or higher number of neutrons. These atoms are called isotopes (from the Greek “same place”). In summary, two atoms are said to be isotopes when:
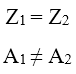
Isotopes having the same number of electrons exhibit the same chemical properties, but not the same physical properties, since their atoms have different masses.
Hydrogen, for example, occurs naturally in three isotopic forms (Figure 5): common hydrogen (or protium) with only one proton and zero neutrons, heavy hydrogen (or deuterium) with one proton and one neutron, and radioactive hydrogen (or tritium) with one proton and two neutrons.
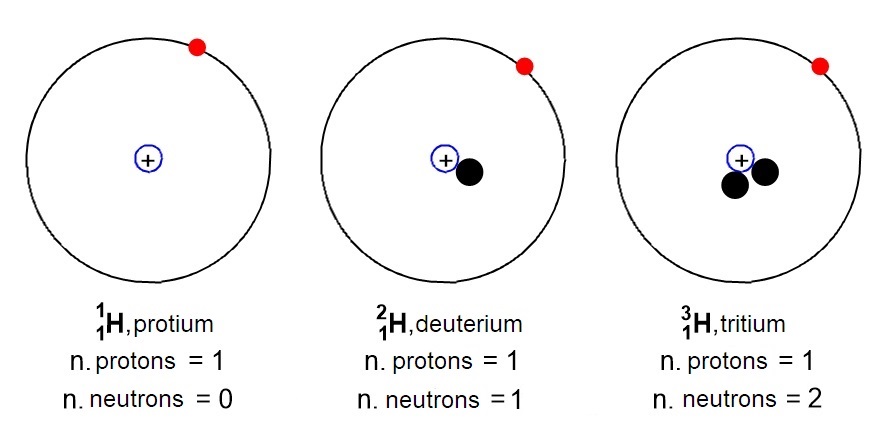
Isotopes are very important because they affect the mass of an atom. In fact, since we have understood that an atom can exist in various isotopic forms, this implies that that particular atom can have different masses depending on how many neutrons will have more or will have less. So how to calculate the mass of an atom, if it varies with the isotopes?
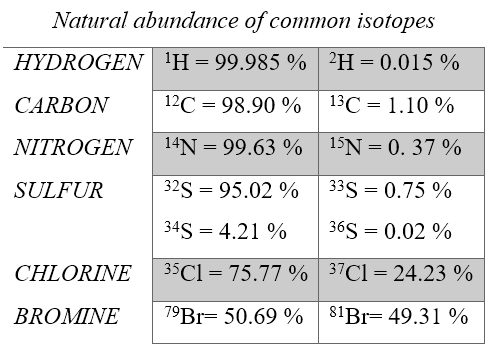
The mass of an atom is calculated as a weighted average (based on isotope abundance percentage) of all the isotopes of that element. Isotopic abundance is expressed as a percentage and therefore equals the number of atoms of a particular isotope out of 100 atoms of the isotopic mixture. For example out of 100 atoms of the element carbon, 98.90 atoms are 12C, carbon 12, and 1.10 atoms are carbon 13, 13C. Examples of isotopic abundances of some elements are given below.
To conclude this article, let’s just give an example of how to calculate the atomic weight of an element. Let’s take carbon as an example, as seen from the table above, carbon exists as 12C with an abundance of 98.90 % and as 13C with an abundance of 1.10 %. However, to calculate the atomic weight of carbon it is necessary to know the weight of each isotope: the weight of 12C is 12.00000 u, while 13C is 13.00335 u. Now it is only necessary to calculate the weighted average of the two isotopes to obtain the atomic weight of carbon:
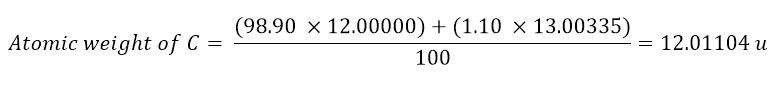
This lesson

Atoms
€
1.50
Download as pdf (unchageable) file

Atoms
€
2.20
Downoald as docx (editable) file