Introduction
In this article, we explore the most common reactions to form esters.
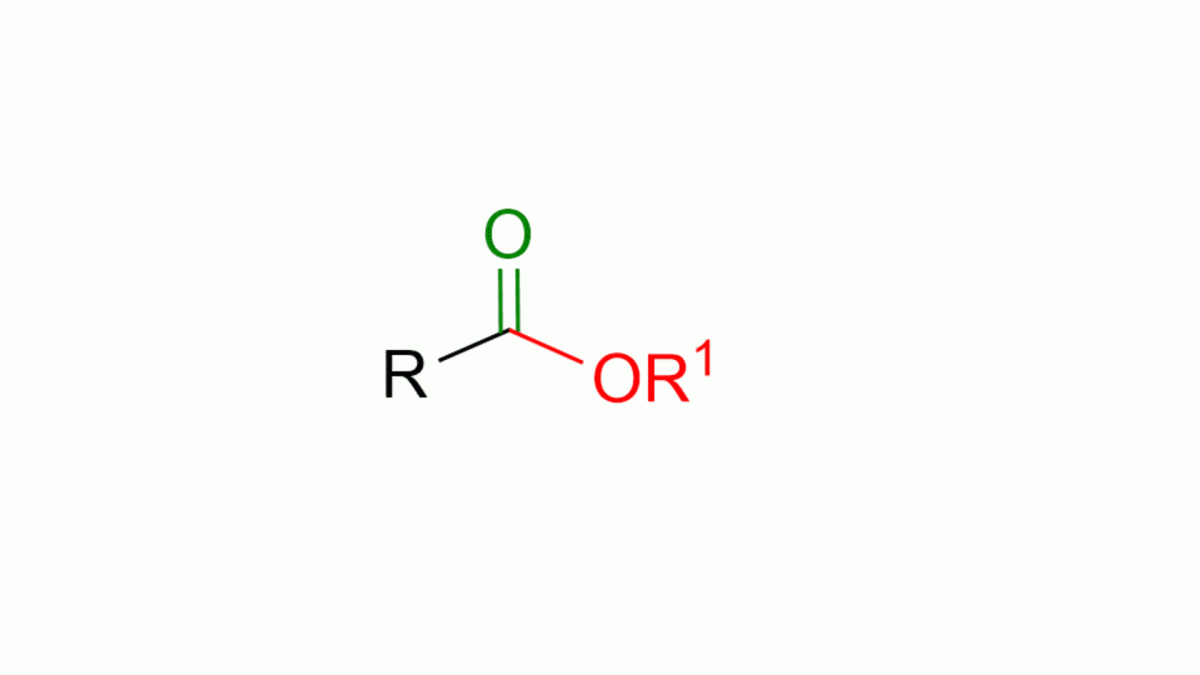
If you don’t remember what an ester is, you can see its structure in the animated figure below.

As seen in the figure, an ester is composed of a carbonyl, C=O, and an alcoholic part, OR1. This part is called “alcoholic” because it derives from an alcohol, R1OH. The ester also has an R part, which is typically an alkyl chain or an aromatic group.
Formation of esters
But let’s get to the heart of our article: how are esters formed?
There are various reactions to form esters, but almost all are characterized by having a carboxylic acid or its derivative (e.g., acyl chloride) as the starting material. This should not seem strange, as esters are also known as derivatives of acids.
Therefore, depending on the reaction conditions (e.g., acidic or basic environment) and the other reactant alongside the carboxylic acid or its derivative, various reactions are known, which we list below.
Fischer Esterification Reaction
The Fischer esterification reaction is certainly the most famous for forming esters. It is characterized by:
Starting materials: carboxylic acid (RCOOH) and alcohol (ROH).
Reaction conditions: acidic catalysis, high temperature.
You can see an example of this reaction below.
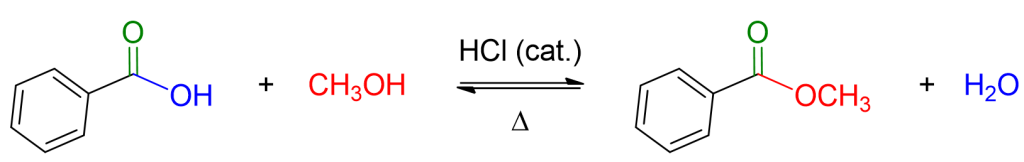
As you can see, the reaction is an equilibrium, implying that it can reverse. Therefore, to drive the reaction towards ester formation, an excess of alcohol needs to be used, which is sometimes employed as a solvent, or water needs to be removed from the reaction environment. The elimination of water can be achieved by utilizing the toluene/water azeotrope and employing a specific apparatus known as a Dean-Stark trap, which allows the removal of water from the reaction environment as it forms.
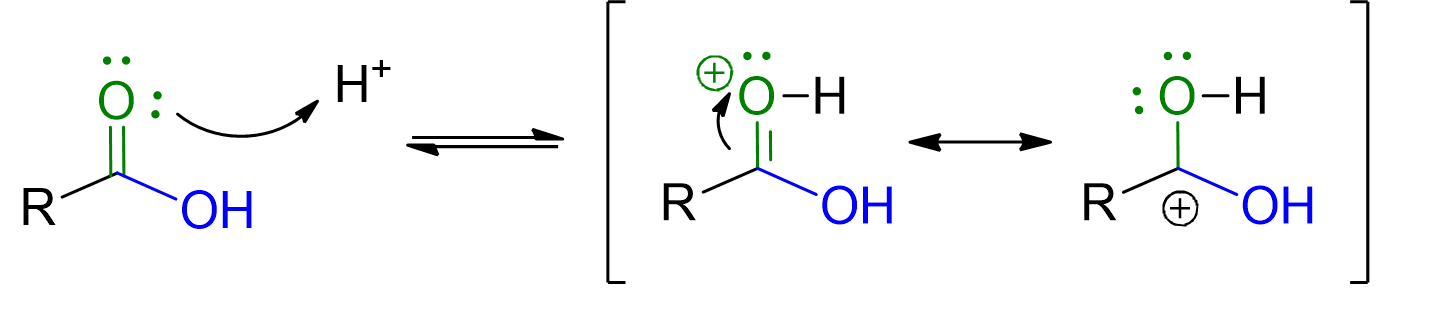
The reaction mechanism is described in the scroll-down tabs below. However, two main points are crucial to remember:
Acid catalysis is employed to make the carbonyl of the carboxylic acid more electrophilic and thus more easily attacked by the nucleophile, namely the alcohol. Additionally, the protonation of the OH group in the carboxylic acid promotes the departure of this group as water. Water, in fact, is a better leaving group compared to OH-.
The oxygen in the alcoholic part of the formed ester comes directly from the alcohol and not from the OH group of the carboxylic acid. This has been proven through isotopic labeling techniques. Therefore, the mechanism involves the addition of the alcohol followed by the elimination of OH as water.
Protonation of the carbonyl group of the carboxylic acid. A resonance-stabilized intermediate is formed, which is more electrophilic.
The alcohol attacks the carbonyl group of the ester, forming a 4-center intermediate.
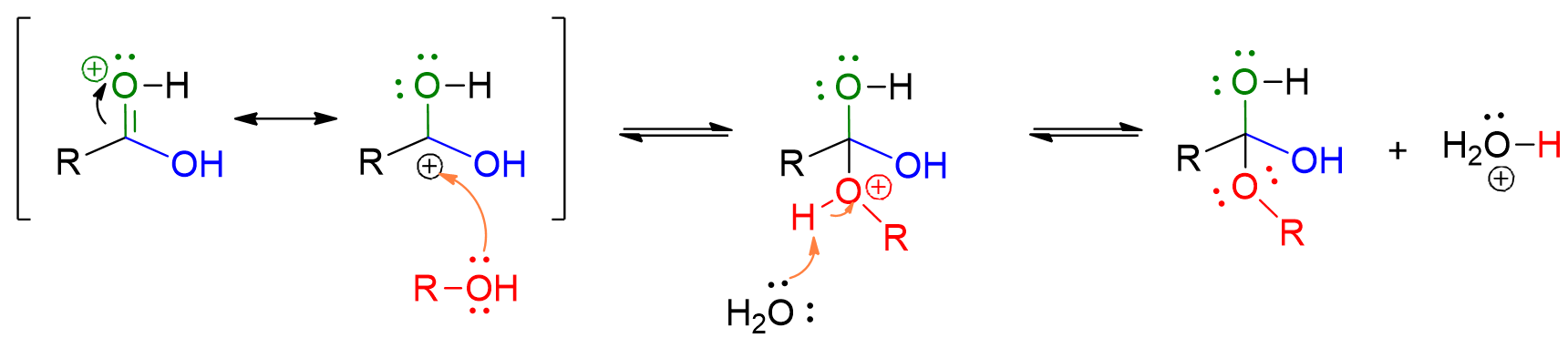
Protonation of the OH group in the carboxylic acid occurs, leading to its departure as water. This results in the formation of the ester.
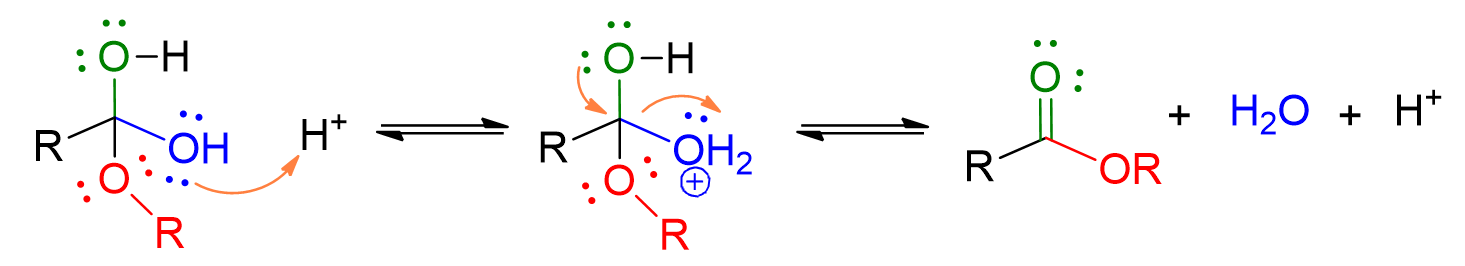
The reactions described in the above mechanism are all in equilibrium! Therefore, it is important to depict all the steps with a double arrow.
In this reaction, the most common acids employed are H2SO4, para-toluenesulfonic acid (p-TsOH), BF3, and they are added in catalytic amounts, meaning a few drops are sufficient. Potentially, any carboxylic acid that is resistant to reaction conditions can be used. However, the alcohols used are generally limited to the simpler ones: MeOH, EtOH, n-PrOH, n-ButOH, etc.
SN2 Reaction
The nucleophilic substitution reaction SN2 involves, once again, a carboxylic acid, but in the form of a carboxylate this time. The counterpart is a primary alkyl halide. Therefore, the reaction involves:
Starting materials: carboxylate anion and primary alkyl halide.
Reaction conditions: polar aprotic solvent (e.g., dimethylformamide, DMF, dimethyl sulfoxide, DMSO).
An example is illustrated in the image below.
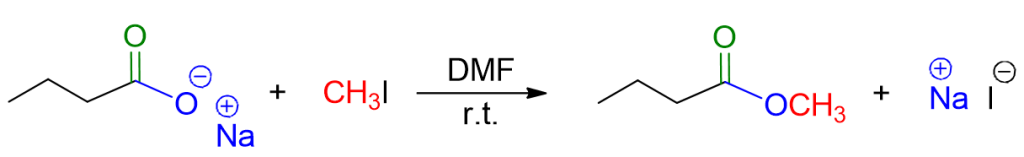
The reaction mechanism is typical of the SN2 bimolecular substitution reaction. It involves the nucleophile, the carboxylic acid, attacking the alkyl halide with simultaneous loss of the leaving group, X–. The reaction occurs in a single stage.
The carboxylate ion, acting as a nucleophile, attacks the electrophile, RX, causing it to lose the leaving group.
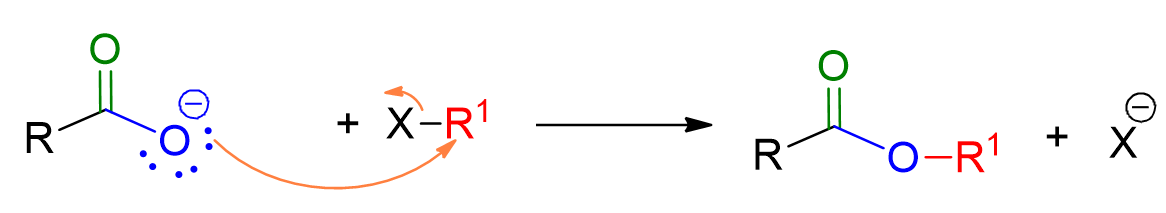
This reaction is performed only with primary alkyl halides to avoid competition with the elimination reaction, as is typical in all SN2 reactions.
Reaction of acyl chloride with alcohol
Another reaction to form esters involves an acyl chloride and an alcohol. Therefore, this time there is no usual carboxylic acid, but there is one of its derivatives, which is also the most electrophilic in the series of acid derivatives and hence the most reactive towards nucleophiles. The acyl chloride, being more reactive, can actually be used to form all acid derivatives. It should not be surprising, then, that it is employed in the ester formation.
Let’s now look at the reaction conditions:
Starting materials: acyl chloride and alcohol.
Reaction conditions: use of a base, typically pyridine, to eliminate the hydrochloric acid formed during the reaction.
For an example, you can see the reaction depicted below.
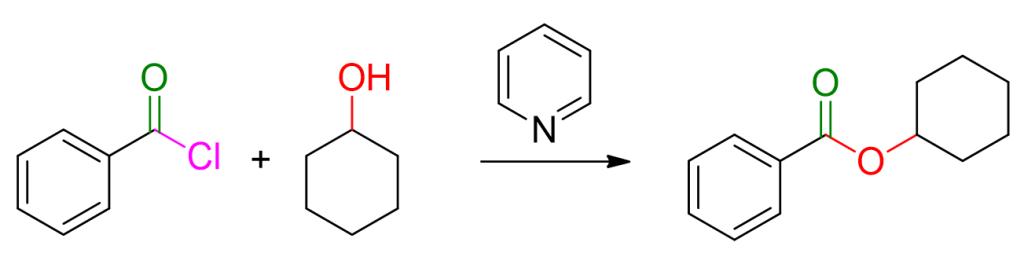
The reaction mechanism involves multiple steps, but they are easily memorable if one considers that, generally, acid derivatives react through the addition of the nucleophile followed by subsequent elimination of the leaving group.
The alcohol, acting as a nucleophile, attacks the carbonyl group of the acyl halide, forming a 4-center intermediate.

At this point, pyridine comes into play to deprotonate the alcohol.
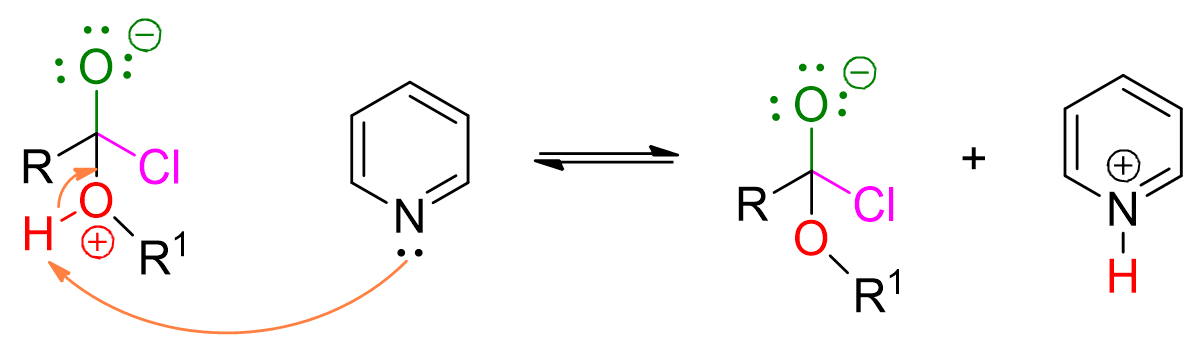
The loss of chloride leads to the formation of the ester. This step is irreversible.
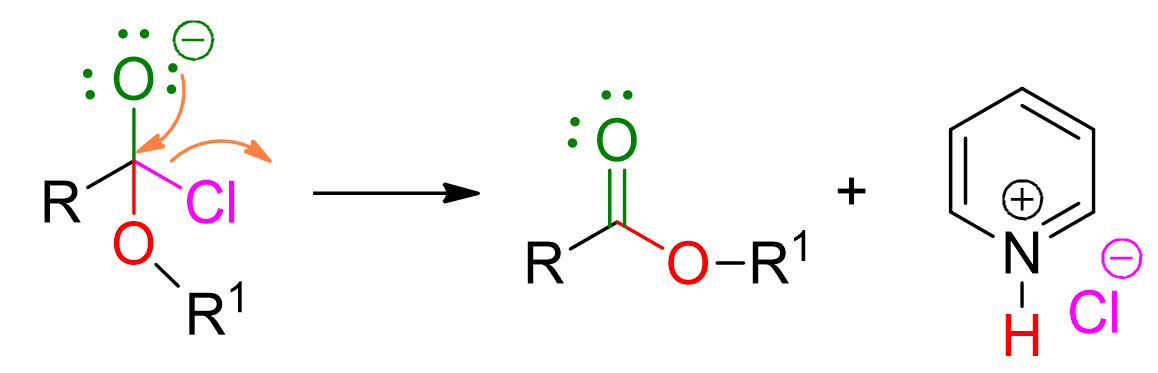
Among the listed reactions for ester formation, this is the most versatile, as it can be carried out with various alcohols and alkyl halides. Moreover, it has the advantage of being irreversible, unlike Fischer esterification. However, it is important to note that in this reaction, primary alcohols react more quickly than secondary alcohols, which, in turn, are faster than tertiary alcohols.

2 replies on “Formation of Esters”
Fanastic blog! Do you have any tips and hints
for aspiring writers? I’m hoping to start my own blogg oon but I’m a
little lost on everything. Would you suggest starting
with a free platform ike Worpress or go for a paid option? There aree soo many
choices out thsre that I’m totally overwhelmed .. Any suggestions?
Appreciate it!
Hello! You can start with the free option, namely WordPress.org. However, it requires some practice to get used to it. If you’re a chemist and interested in joining our website, why not consider joining our platform?
Would you like to know more? Feel free to email us at info@operachem.com, including your motivation and skills. We’d love to hear from you!