Introduction
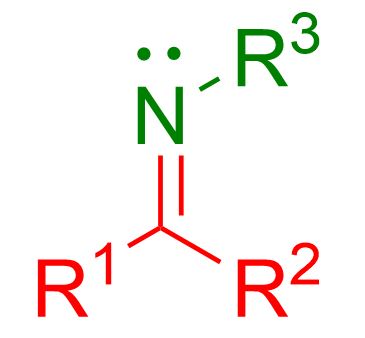
In this article, we’ll explore the most common reactions used to form imines. If you’re unsure what an imine is, refer to the image below for clarification.
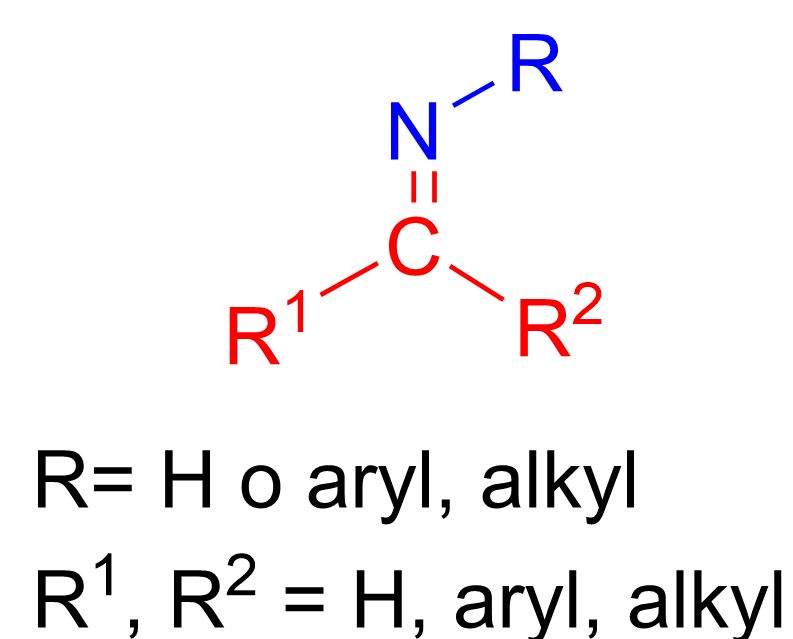
As shown in the figure, an imine is characterized by a double bond between carbon and nitrogen, C=NH, or, in most cases, C=NR, where R can be any alkyl or aromatic group.
Starting Materials
To master the formation of imines once and for all, it can be helpful to understand the starting materials, which can be achieved by simply examining their structure.
We have just explained that imines are characterized by a double bond between C and N, C=N. If we consider breaking this bond, we obtain the building blocks from which an imine is formed (see figure below). The first red piece is a carbon bearing a double bond. The simplest possibility now is that this carbon is bonded to an oxygen atom. In this way, we have already created the first class of compounds needed to form an imine—namely, aldehydes and ketones. The second class can be derived from the blue fragment. This fragment is N-R; if we complete the nitrogen valence with hydrogen atoms, we obtain a primary amine.
Therefore, to form an imine, the starting materials required are an aldehyde or a ketone and a primary amine. It’s important to emphasize that the amine must be primary; otherwise, a different product would form.

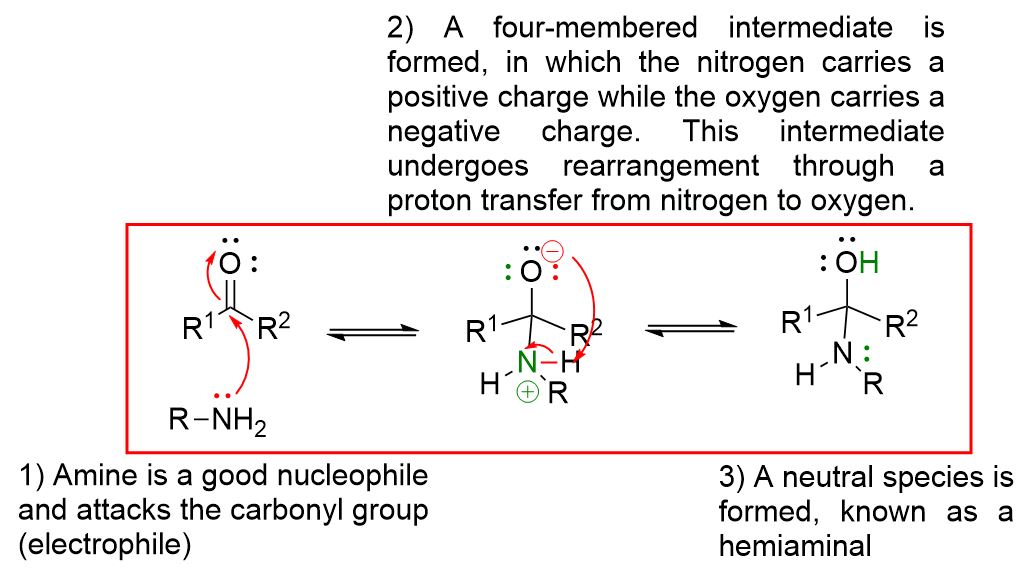
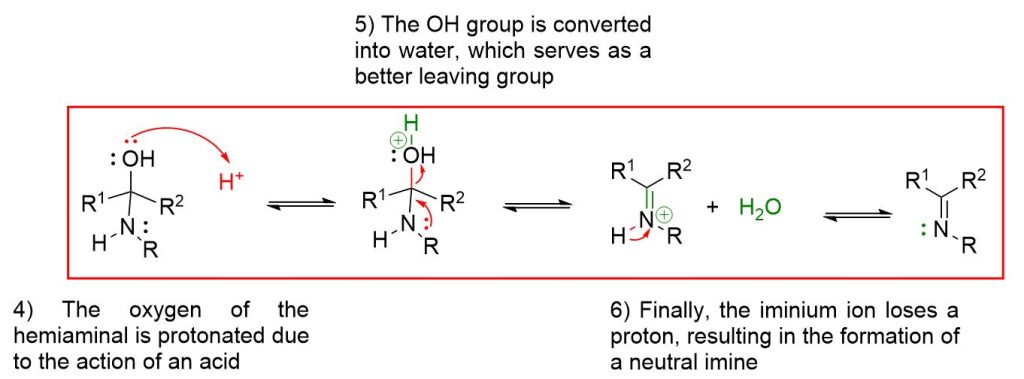
The reaction for imine formation
The reaction to form imines occurs through steps that are all reversible. You can view each step by clicking the dropdown buttons below.
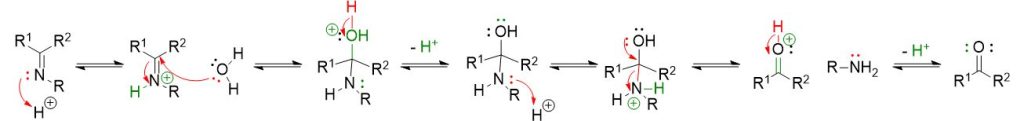
As can be seen from the mechanism described above, the first step involves the nucleophilic attack of the primary amine on the carbonyl to form a neutral four-center intermediate called a hemiaminal.
In the second step, however, an acid is introduced to facilitate the elimination of the OH group, as water, and lead to the formation of the imine. Furthermore, since all the described steps are equilibrium processes, it is necessary to shift the reaction to the right for complete conversion.
In summary, for the formation of an imine to be possible, the following conditions must be met:
Acid catalysis is required to favor the reaction in the second stage through protonation of the hydroxyl group, converting it into water, which is a better leaving group compared to OH⁻;
The removal of water formed in the reaction environment is necessary. This is required to shift the equilibrium to the right and prevent the regeneration of the reactants through the hydrolysis of the imine (reaction of the imine with water to yield the carbonyl compound and the primary amine again).
pH of the reaction
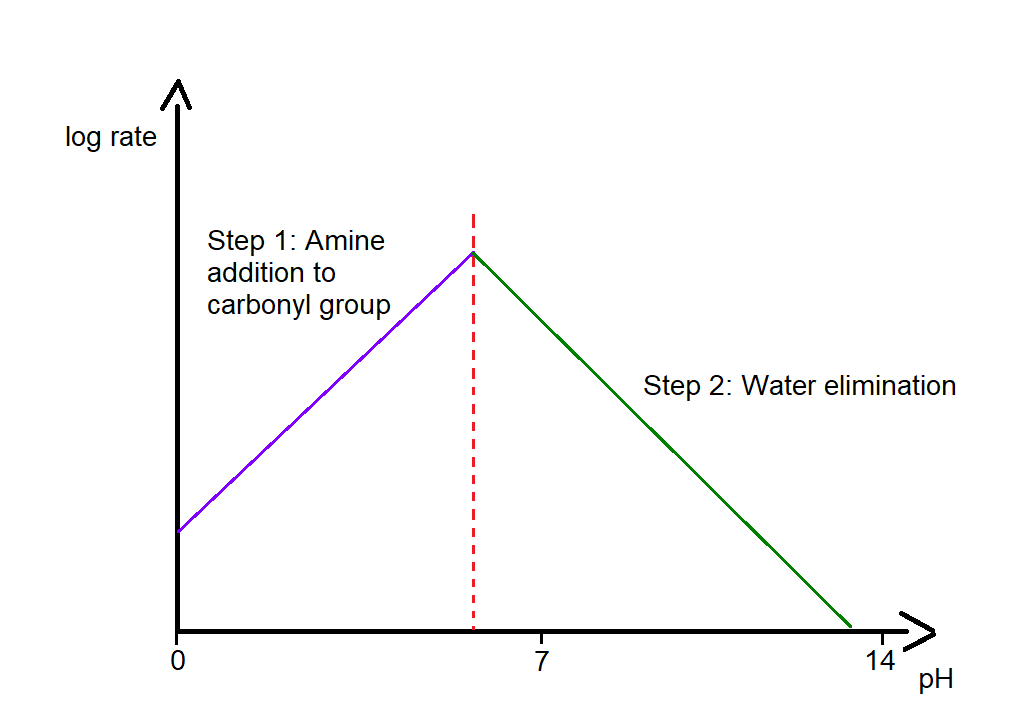
As we have seen in the mechanism, the first stage of the reaction occurs under neutral pH conditions. However, in the second stage, acid catalysis is required to drive the equilibrium toward the products.
Specifically, it has been observed that the reaction proceeds faster when the pH is in the range of 4-6. At more acidic pH values, below 4, the reaction slows down because, at such pH levels, the primary amine is fully protonated, which decreases the rate of the first reaction stage (see figures below). At higher pH values, above 6, the concentration of H⁺ ions is too low to favor the protonation of the OH group, thereby slowing down the second stage of the reaction.
Removal of water
As we have seen, the reaction to form the imine is an equilibrium reaction. Therefore, one way to drive the reaction to completion is by removing water from the reaction environment. This can be achieved using the following techniques:
Use of a Dean-Stark trap;
Use of hygroscopic salts;
Use of molecular sieves.
The first method involves using a special apparatus known as a Dean-Stark trap, which you can see in the image below.

This trap is a glass apparatus consisting of a longitudinal tube and an oblique lateral tube. The longitudinal tube is made up of an upper end, to which a cooler (or condenser) can be attached, and a lower end with a faucet. The oblique tube of the Dean-Stark trap serves as the connection to the reaction flask.
The operation of the trap is based on the use of an azeotropic mixture, such as toluene/water, in which the reaction takes place. Specifically, the reaction is carried out under reflux, heating the mixture to the boiling point of the toluene/water azeotrope. In this way, the mixture evaporates, and through the lateral arm of the Dean-Stark trap, the vapors are directed toward the cooler, which condenses them and allows them to fall into the lower part of the trap. Here, the two solvents separate into two distinct phases due to their different densities: water forms the lower phase, while toluene forms the upper phase. In this way, the water can be removed through the faucet, while the toluene returns to the reaction flask. The reaction is considered complete when all the expected water has been collected and removed from the reaction environment.
If a Dean-Stark trap is not available, the reaction can be carried out by adding hygroscopic salts to the reaction flask, which are salts capable of absorbing the water as it forms. The most commonly used salts in this case are Na2SO4, MgSO4, silica gel, etc
A similar alternative is the use of molecular sieves, which are materials based on zeolites with selective porosity for the removal of small molecules. Molecular sieves used for water capture have pores with a diameter of 3-4 Å (commercially known as 3A and 4A).
Imines stability
As we have repeated several times in this article, the imine formation reaction is reversible. This means that once imines are formed, if they encounter water, they can easily hydrolyze and revert to a primary amine and a carbonyl compound. This hydrolysis often occurs even without basic or acidic catalysis. The hydrolysis reaction is the reverse of imine formation and is shown below.
In general, we can say that the most stable imines are those where either the carbon or the nitrogen atom carries an aromatic group. These types of imines are stable enough to be isolated. On the other hand, imines derived from ammonia cannot be isolated because they are very unstable; however, they can be detected in solution. Finally, very stable imines are those in which the nitrogen atom carries an electronegative group: this is the case for oximes, hydrazones, and semicarbazones, which you can see in the figure below.
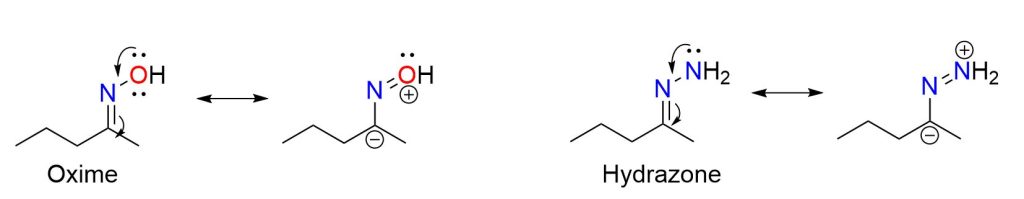
The stability of such imines arises from the fact that the group attached to the nitrogen participates in the delocalization of the C=N electrons, creating a resonance structure with a negative charge on the carbon, which thus becomes less susceptible to nucleophilic attack.
Conclusion
In this article, we have seen how to form imines. The reaction is reversible and requires the removal of water to drive it towards the products. Additionally, the ideal pH for the reaction is between 4 and 6. If you’re curious to see how imines are actually created in the laboratory, you can take a look at our article that describes the typical procedures carried out by our scientists.