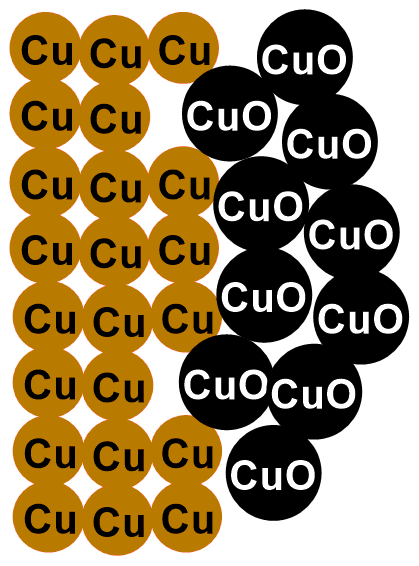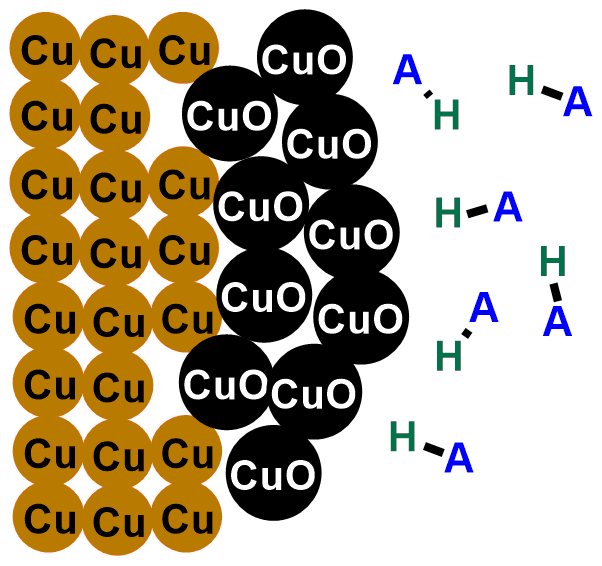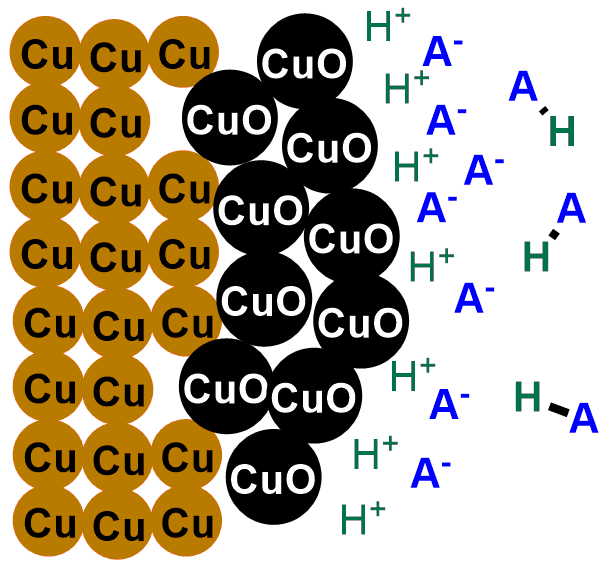Chemicals and Equipment
To clean your old coins, you will need the following chemicals and equipment:
Chemicals
Equipment
Time
Lemon
(or vinegar)
Water
Blackened coins
Glass
Tweezers
5 min
As seen in the table, you will need a lemon and/or vinegar, along with a bit of water. Regarding the equipment, you will need what you typically find at home: a glass, tarnished coins and a pair of tweezers.
The experiment takes very little time, so it’s suitable even for the laziest.
Instructions
The operations to be performed are very simple:
- Take a glass and fill it with water.
- Squeeze a lemon into the glass with water.
- Immerse your tarnished coins in it for a few seconds.
- Get the coins back with tweezers and dry them with a paper towel.
You’ll notice, now the coins are shiny as if freshly minted!
If, instead of lemon and water, you choose to use vinegar, remember that in this case, you’ll need to immerse your coins for a longer time before seeing them clean.

Would you like to download this experiment and have all the information with you? You can download our pdf or docx files here.
Chemical explanation
How coins are made
The 1, 2, and 5 euro cent coins are made of 95% steel and have an outer plating of copper, which constitutes the remaining 5%.
It is indeed the copper that gives our coins the reddish appearance that distinguishes them. However, as we know, coins over time lose their shiny and vivid appearance and take on a faded and dull color, to the point of becoming completely black in more severe cases. So, we ask ourselves, ‘Why do our coins tend to tarnish?


The Tarnishing Reaction
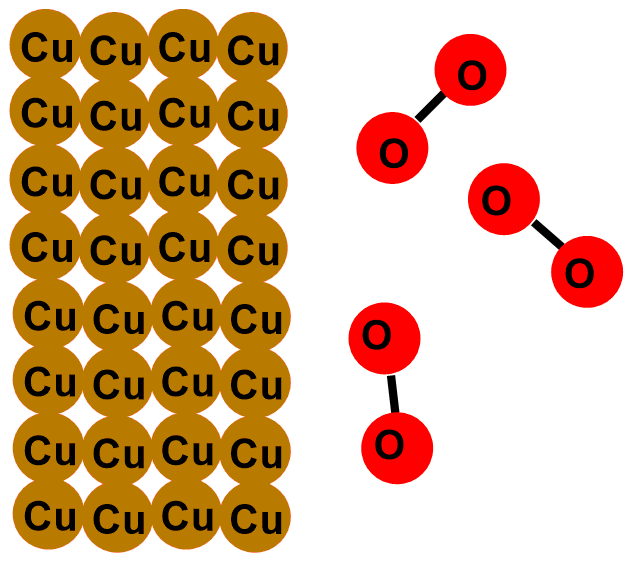
Coins tarnish because copper (Cu), which forms the outermost layer of the coin, tends over time to react with the oxygen (O2) in the air, following the reaction:
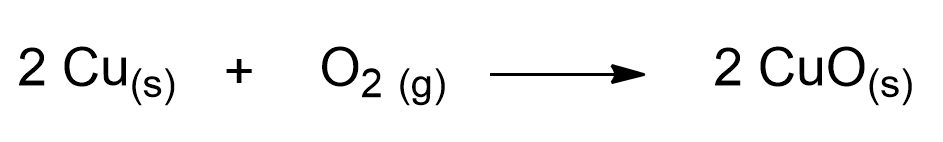
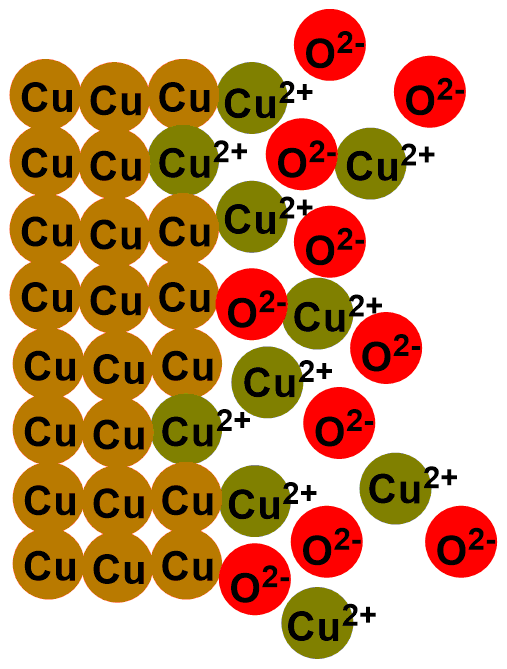
This is a reaction known as redox reaction. Specifically, copper donates its electrons to oxygen and transforms into copper (II) oxide (CuO). This compound is a black solid that layers on our coins. That’s why they tarnish over time!
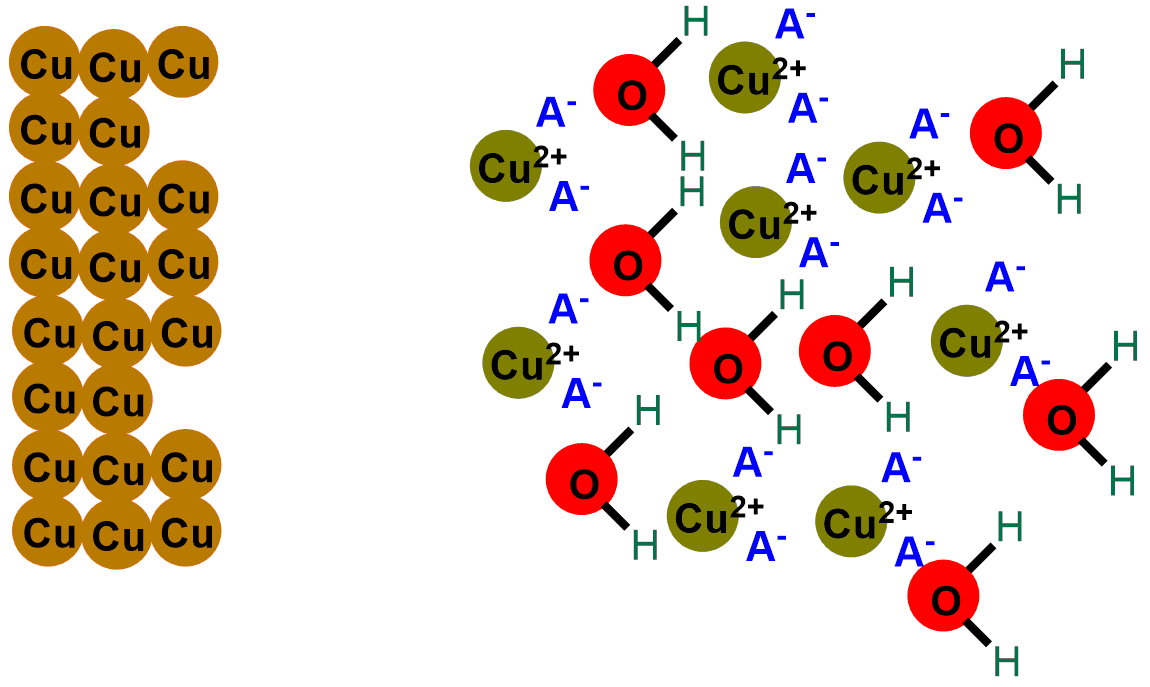
In the images below, you can see the depicted reaction just described. The first image illustrates the outermost layer of the coins, which when new is composed only of copper (Cu). Subsequent images show the formation of copper oxide (CuO) through the reaction with oxygen (O2).
The Cleaning Reaction
How do we then clean our coins marred by black copper (II) oxide (CuO)?
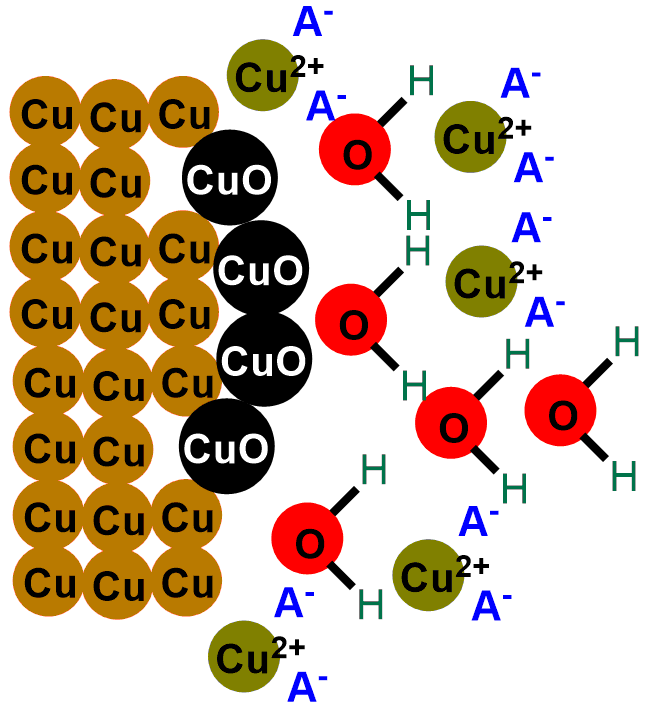
We need a substance that destroys it. This substance is any acid. If you don’t know what an acid is, don’t worry; in fact, it’s enough for you to know that an acid, HA, is a substance that, in solution, releases H+ ions and A–, where H+ is the positively charged hydrogen ion, and A– indicates any negatively charged species, which will vary depending on the acid used. For example, for hydrochloric acid, HCl, A– is the Cl– ion, for sulfuric acid with the formula H2SO4, A– is SO42-, and so on. You will have noticed, therefore, that all acids have in common the H+ ion they release in solution.
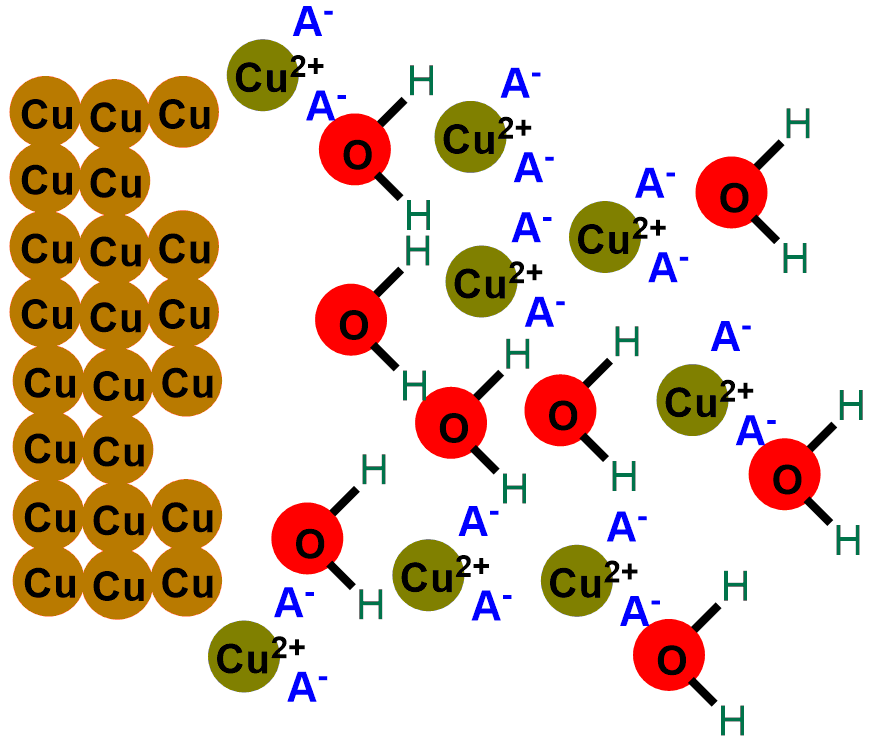
So, when we put our coins in acid, the H+ ions released by the acid react with the oxygen in copper (II) oxide (CuO), transforming it into water. Meanwhile, the A– ion captures the Cu+ ions, bringing them into solution. You can see the reaction describing the phenomenon below:
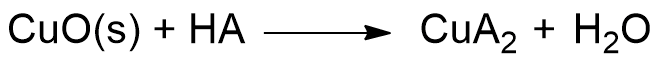
In short, what an acid does is gradually eat away the layer of black copper (II) oxide (CuO), revealing once again the shiny metallic copper, initially covered by the oxide. If you scroll through the images below, you can see the depiction of the coin-cleaning reaction.
The specific reactions for lemon and vinegar
In the experiment mentioned in this article, we used lemon or vinegar. Why? Because both are acids.
Indeed, lemon contains citric acid, whose formula is visible below.

Citric acid reacts with copper oxide, forming copper citrate and water, according to the reaction:

Vinegar, on the other hand, is an aqueous solution that contains acetic acid, CH3COOH. This acid reacts with copper oxide to form copper acetate and water, according to the reaction:

However, vinegar takes more time to clean our coins, while lemon is quite immediate. Why? Because the acetic acid in vinegar is less acidic than citric acid, and it is also present in lower quantities in vinegar.
Conclusion
With this experiment, we have learned two key points:
- Our 1, 2, and 5 cent euro coins are composed of an outer layer of copper (Cu), which, due to exposure to oxygen, tends to darken over time. This process results in the formation of a layer of copper oxide (CuO), a black solid.
- We can clean our coins using an acid, such as citric acid from lemons or acetic acid from vinegar. These acids work by slowly dissolving the black oxide layer, allowing the metallic copper underneath to resurface and regain its shine. As a result, the coin becomes bright and shiny once again.
Did you enjoy this experiment and our explanation? Let us know by voting on this post below or leaving a comment. Additionally, if you’re curious to see what happens to coins in a powerful acid, watch the video below.
This lesson

Clean coins with chemistry
€
1.50
Download as pdf (unchageable) file

Clean coins with chemistry
€
2.20
Downoald as docx (editable) file