Introduction
Ullmann coupling reaction was studied by Ullmann at the beginning of 19th century. The first publication by Ullmann on his coupling reaction has been included in our collection of unmissable publications (you can read it here).
.
The Ullmann coupling showed for the first time how a transition metal catalyst, in this context Cu, can catalyze the C-C bond formation between 2 aryl groups.
.
In this article, we are going to learn what Ullmann coupling is, which products are obtained and the mechanism of the reaction.

Want to read this article offline or save it for personal use? Download it in DOCX or PDF format here.
Ullmann homocoupling
At the beginning of his trials, Ullmann used stochiometric amount of Cu as catalyst and high temperature condition, to make an homocoupling reaction of halobenzene derivatives. In the following example, you can see a typical Ullmann homocoupling reaction:

In this reaction 2-bromonitrobenzene reacts with itself to give a C-C bond formation (green line) by breaking the C-Br bond. As you can see this is an homocoupling reaction because the 2-bromonitrobenzene react with itself.
But how does this reaction occur? Let’s the mechanism.
Mechanism of Ullmann homocoupling
The mechanism of Ullmann coupling is depicted in the Scheme below.
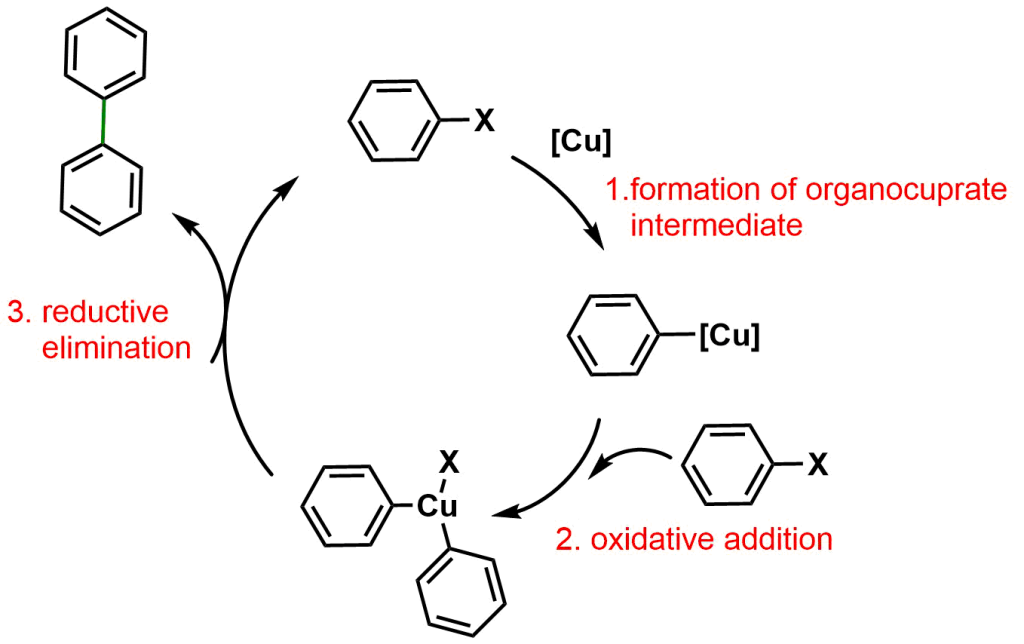
The first step involves the formation of an organocuprate intermediate Ar-Cu. The addition of another Ar-X molecule via oxidative addition affords the Ar2-CuX complex which undergoes reductive elimination to provide the coupling product Ar-Ar.
Ulmann cross-coupling
Besides investigating homocoupling reactions, Ullmann also explored cross-coupling reactions to synthesize amines and ethers. The same methodology used for homocoupling can be extended to these transformations: by mixing an aryl halide (Ar-X) with an aryl amine (Ar-NH₂) in the presence of a stoichiometric amount of copper (Cu), an N-C bond is formed, yielding a diaryl amine (Ar-N-Ar). This discovery was done by Ullmann in 1903 with the following reaction:
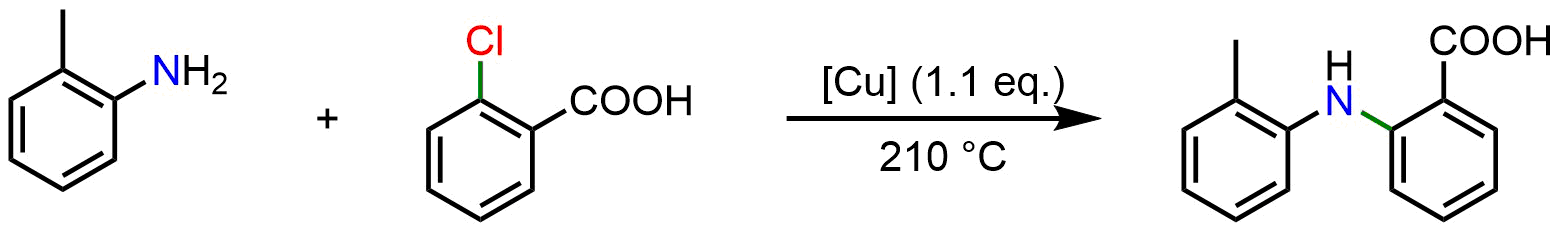
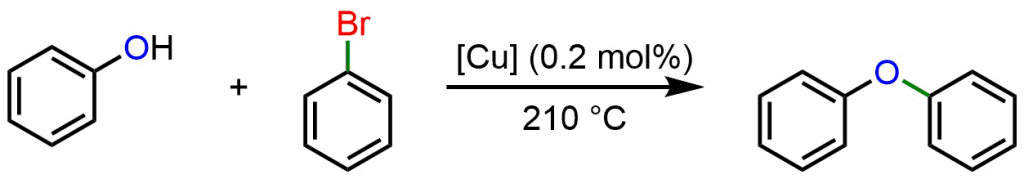
Similarly, in 1905 Ullmann demonstrated how reacting an aryl halide (Ar-X) with an aryl alcohol (Ar-OH) in the presence of catalytic amount of Cu, results in the formation of a C-O bond, producing a diaryl ether (Ar-O-Ar).
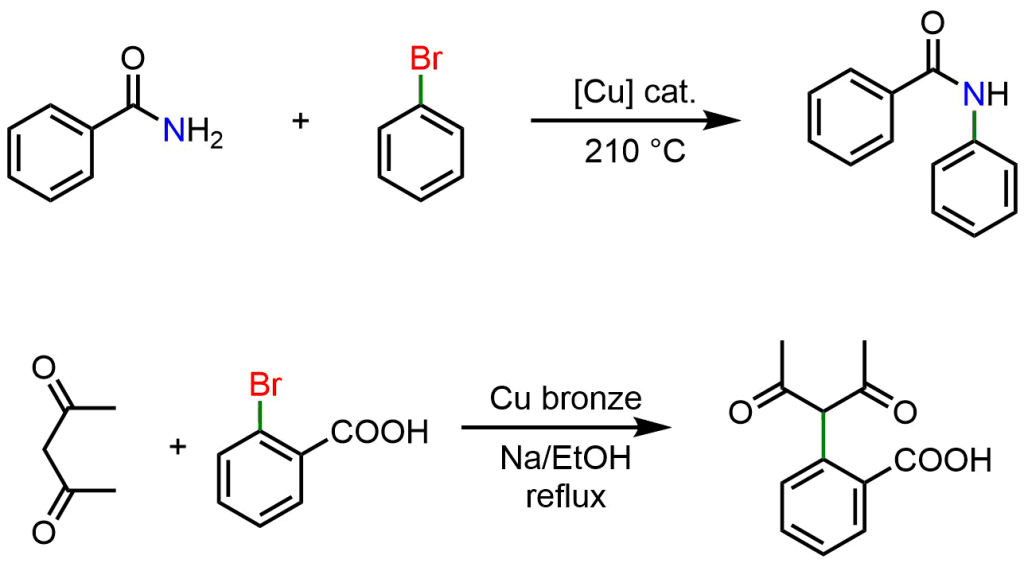
It was clear then that Cu could catalyze the reactions of aryl halides (Ar-X) with a generic nucleophile (Nu). Therefore, other examples of Ullmann cross-coupling involve the use of nucleophiles such as amides and enolates.
What type of copper should be used?
For Ullmann coupling, various sources of copper have been successfully used, ranging from metallic Cu(0) to various Cu salts, including Cu(I) and Cu(II). However, multiple studies suggest that the true catalytic species are Cu(I), which may be present as impurities in metallic copper or generated in situ through oxidation by atmospheric oxygen.
It has also been hypothesized that when Cu(II) is used as a catalyst, Cu(I) species can form through the reduction of Cu(II) by coordinating nucleophiles present in the reaction, such as amines or phenoxides.
Therefore, the specific form of copper used in Ullmann coupling appears to be less critical, as oxidation/reduction processes during the reaction inevitably lead to the formation of the active Cu(I) species.
Hence, are you worried about which type of copper to use? Don’t be! Just use any source of Cu you have in the lab!
What’s new in Ullmann coupling?
Nowadays, the Ullmann coupling is commonly referred to as the Ullmann-Ma coupling. This is because Ma made a significant contribution to this reaction starting in the early 2000s when he discovered that amino acids can act as ligands in Cu-catalyzed reactions, leading to the following advancements:
Reduction of catalyst loading – Before this discovery, the Ullmann reaction required a stoichiometric amount of catalyst or generally high catalyst loading.
Milder reaction conditions – Thanks to these ligands, the reaction temperature was significantly lowered, dropping from the typical 200–300 °C of standard coupling reactions to 40–100 °C when using a suitable ligand.
The first generation of ligands developed by Ma consisted of amino acid-based ligands, which helped reduce the reaction temperature. The figure below illustrates some examples of these ligands, along with the reactions in which they were used.
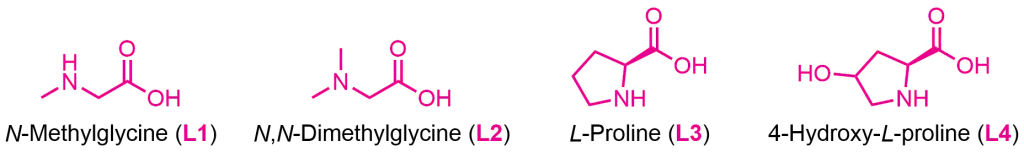
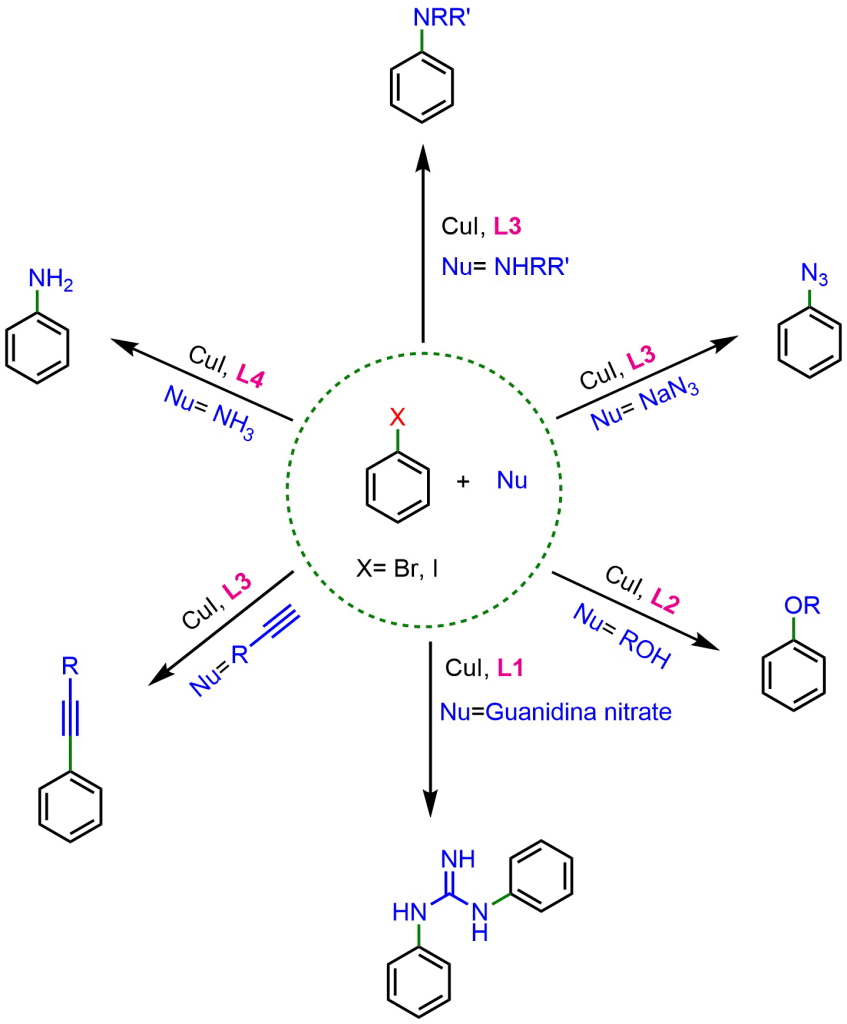
However, from 2012 onward, Ma developed a second generation of ligands employing oxalic diamides and general amides. This new class of ligands drastically reduced Cu loading and significantly expanded the coupling scope, even enabling the reaction with less reactive aryl chlorides. Some of the most interesting second-generation ligands are shown below.
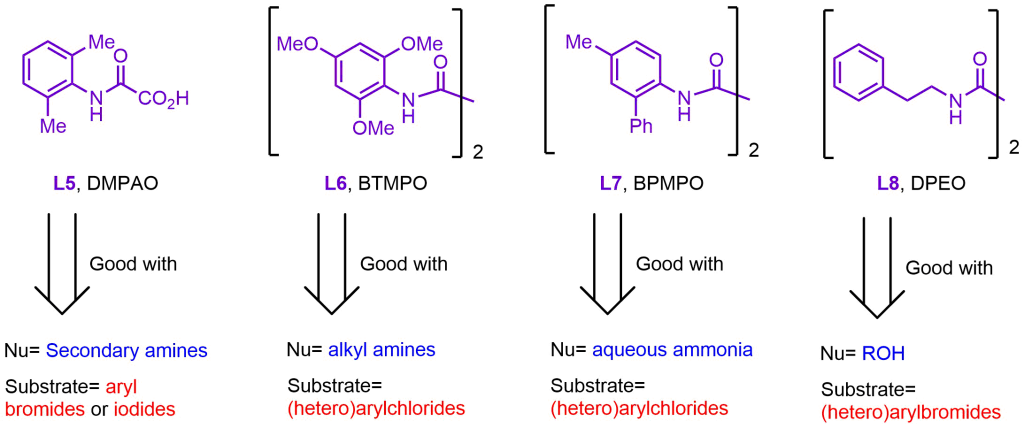
Conclusion
In this article, we have explored what Ullmann coupling is, how it works, and the latest advancements. In particular, we have discussed that:
Ullmann coupling involves Cu as a catalyst and can be used for both homocoupling reactions and cross-coupling with a nucleophile (e.g., amines, alcohols, etc.).
Today, Ullmann coupling is also known as Ullmann-Ma coupling due to Ma’s significant discovery regarding the use of ligands to reduce catalytic loading and enable milder reaction conditions.
This lesson

Ullmann coupling-An overview
€
1.50
Download as pdf (unchageable) file

Ullmann coupling-An overview
€
2.50
Downoald as docx (editable) file
