Introduction
You are at the beginning of your studies and you need to know what alkanes are, but does organic chemistry scare you? Well, this article is for you, because with simple words and a few chats we will see the basic concepts to know for your exam.
What are alkanes?
Alkanes are the simplest of organic molecules, made up only of hydrogen, H, and carbon, C, linked together by simple bonds. They are defined as
molecules with the molecular formula CnH2 (n+2)
where n is any integer. Therefore, if n=1, the formula we get is CH4, methane, the simplest molecule in organic chemistry. Proceeding with the number n, we obtain the other alkanes following methane:

As can be seen from the figure, the name of the first 4 alkanes is given by the root MET-, ET-, PROP-, BUT- + the suffix –ANE which indicates alkane. Unfortunately, these roots have to be memorized, because there is nothing that really links them to the structure of the molecule, except the suffix -ANE, which is characteristic of alkanes. However, the good news is that the names of the later alkanes are easy to derive. In fact, the Greek numeral prefixes (pent-, ex-, ept-, ott-, non-, dec-, etc.) are used to indicate the number of carbon atoms present, followed by the usual suffix –ANE (see Figure 2 and Table 1).


Geometry of alkanes
Carbon in alkanes always forms 4 single bonds, respecting its valence equal to 4. Hydrogen, as usual, instead makes only one bond, having valence 1. Remembering the valence can be useful for correctly writing the structural formulas of these compounds. Nevertheless, let’s go deeper into understanding how carbon binds. Let’s take the external electron configuration of carbon:
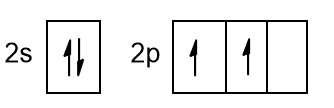
From figure 3, we learn how carbon has 2 paired electrons in the 2s orbital and 2 unpaired ones in two p orbitals, while the last p orbital is empty. With this configuration, carbon could only make two bonds, sharing the unpaired electrons of the p orbitals or at most, it could make 3 bonds if it also uses the empty orbital to accept a pair of electrons from an external atom. We immediately realize that this configuration does not explain in any way the valence 4 of carbon in alkanes. To explain this phenomenon, chemists have introduced the concept of orbital hybridization.
In particular, according to this theory, one of the 2s electrons is promoted into the empty 2p orbital (Figure 4), slightly destabilizing the atom.
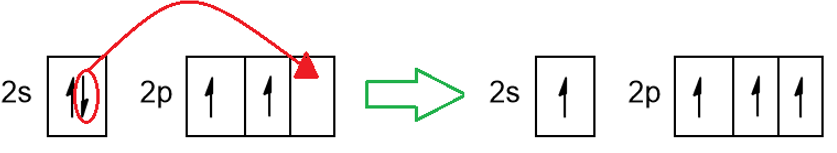
Thanks to this promotion, there are now 4 unpaired electrons in the four orbitals. These orbitals are now mixed together to form another 4 orbitals called sp3 hybrids. They are orbitals with characteristics halfway between those of the p (peanut-shaped) orbital and those of the s (spherical) orbital (see Figure 5). A kind of fusion has taken place, like the one that between Goku and Vegeta gave life to a hybrid being Vegekou, for the lovers of the Dragonball television saga. These hybrid orbitals are ready to bind 4 other external atoms (other carbon and/or hydrogen atoms) so that the carbon acquires the stability given by the achievement of the octet. The bonds will all be single bonds known with the term σ bonds. The molecular geometry of a sp3 hybrid carbon is the tetrahedron with 109.5° angles between bonds (Figure 5).

Thanks to the hybridization theory, we now know exactly the molecular geometry of all alkanes (tetrahedral geometry), all bond angles (always 109.5°) and bond types (all single bonds, called σ). Let’s take an example, let’s take methane, CH4, we know that carbon is sp3 hybridized and that its orbitals will arrange themselves at the vertices of a tetrahedron to bind the 1s orbitals of 4 hydrogens (Figure 6). The bond angles are all 109.5°. Similarly, in ethane, C2H6, the two carbons are sp3 hybrids and will bind each other via a single σ bond. The carbon atoms will also link as many hydrogens as necessary to react all of the remaining hybrid orbitals (Figure 6).

Similar reasoning can be used to construct higher order alkanes (propane, butane, etc.).
Structural isomerism
From general chemistry, you know that structural isomers are molecules with the same molecular formula, but different structural formula. In organic chemistry, this topic is not just a notion suspended in the air, but it is an important reality for laboratory activity. In fact, alkanes can present this type of isomerism starting from butane. Methane, ethane, and propane have no structural isomerism, because their atoms cannot connect differently.
Before moving on to the isomers, let’s see a concept that can help us identify them: the nomenclature of the carbons of the alkyl chain. We need to distinguish between:
- Primary carbons (CH3-), those external to the chain that bind only 1 other carbon atom (Figure 7);
- Secondary carbons (-CH2-), those internal to the chain that bind 2 other carbon atoms;
- Tertiary carbons (-CH-), those internal to the chain, but which bind 3 carbon atoms.
- Quaternary carbons (-C-), internal to the chain, which bind the maximum possible number of carbon atoms, i.e. 4. These carbons obviously cannot bind any hydrogen, having saturated the valence.
Now we can find the isomers. Let’s start with butane, the first of the alkanes to show structural isomerism. This compound has the molecular formula C4H10, however it is possible to write two isomers with the same molecular formula:
- normal butane (abbreviated to n-butane) with a straight-chain;
- isobutane (abbreviated to i-butane) with branched-chain..

In figure 8, you can see that normal butane is characterized only by primary and secondary carbons and has a linear chain, as best seen by the ”skeletal” structural formula below which reports only the carbon-carbon bonds, omitting the rest. Isobutane, on the other hand, has 3 primary carbons and one tertiary carbon; the chain is less linear, as can be seen better from the skeletal structure formula below. What has changed from normal butane to isobutane is the position of a CH3 that in i-butane has shifted to bind an internal carbon, generating a tertiary carbon. Isobutane can also be viewed as a propane with a methyl substituent on the inner carbon, in fact the IUPAC name of this compound is 2-methylpropane.
Obviously, the number of structural isomers will be greater the longer the alkyl chain is. To learn how to write isomers, it will be enough to first write the linear chain isomer, the simplest, and then build the others, taking external pieces of the linear isomer (e.g. a CH3, a CH2-CH3, etc.) and binding them to internal carbons until all possibilities have been exhausted. To know if the work has been done correctly, check that the type of carbon (primary, secondary, tertiary, quaternary) has actually changed from one isomer to another.
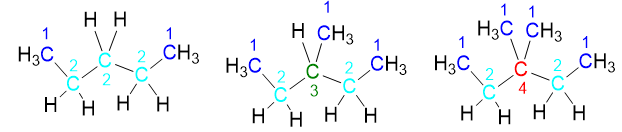
Let’s take a last example by taking C5H12 pentane, the next molecule after butane, We first write the linear isomer, which will have only secondary and primary carbons (see number 1 and 2 in the picture below). This isomer is the normal pentane, n-pentane

If I take an external -CH3 from this isomer and move it onto the internal -CH2– with the progressive number 3 (see black numbers), I will obtain the second structural isomer: isopentane, i-pentane or 2-methylbutane. As can be seen in the figure, this isomer has a tertiary carbon, in addition to secondary and primary carbons, while normal pentane had only secondary and primary carbons. Therefore, since the type of carbon atoms has changed, we are sure we are dealing with an isomer.

However, it is not over yet, in fact if we take an external methyl to isopentane, for example methyl n. 4 (see numbers in black) of the chain and we move it on carbon n.2, we obtain a new structure (Figure 11). This structure is in fact a new isomer (neopentane) because it has only primary carbons and a quaternary carbon, a very different situation from isopentane.
Passing from the linear isomer to the branched ones, we notice how the main linear chain gradually gets shorter; in fact we have gone from a chain with 5 carbon atoms (see numbers in black in the figures) in n-pentane to one with 4 atoms in i-pentane, up to only 3 atoms for neopentane.
We have therefore seen the strategy for writing the isomers, however to understand better you will necessarily have to train. For example, try writing the structural isomers for hexane and take OUR FREE QUIZ

Physical properties of alkanes
Boiling and melting points
The first four alkanes (from methane to butane) are gases, while pentane is a low-boiling liquid (b.p. = 36°C). Alkanes from pentane to heptadecane (C17H36) are liquids; instead, from octadecane (C18H38) onwards they become solid.
From this trend, it is easy to understand that as the chain lengthens, the boiling points increase. In fact, the longer the alkyl chain, the greater the Van der Waals interactions between one chain and another. See the table below.

The presence of branching lowers the boiling point because branching makes the contact area between one molecule and another smaller, reducing the Van der Waals attractions. As an example, consider the isomers of pentane (Figure 12). You can see that the more branched neopentane is a gas at room temperature, while the straight-chain n-pentane is a low-boiling liquid.

As far as the melting points are concerned, the trend is not as regular as for the boiling points. However it is possible to see a trend if we distinguish between alkanes with even number of carbon atoms and alkanes with odd number of carbon atoms.
Alkanes with even numbers of carbon atoms in the crystalline state show a tighter packing than that of alkanes with odd numbers, thus having higher melting points. In fact, their compact packing improves the Van der Waals interactions. Conversely, alkanes with an odd number of carbon atoms have lower melting points
In both series, however, there is an increase in melting points with increasing chain length, as for boiling points. Branching generally lowers melting points, with the exception of those branching that lead to a more symmetrical structure, in which case the melting point increases. Let’s look at the melting points of the pentane isomers: Isopentane, which has branching, has a lower melting point than ordinary pentane, but neopentane has the highest melting point of the series. This is because the two branches present lead to a symmetrical structure, like a ball, which packs better in the crystalline lattices of the solid..

Density
Alkanes are the lowest density compounds; in fact, they have a much lower density than the water on which they float (see Table 2).
Solubility
Using the rule of ”like dissolves like”, it can easily be deduced that alkanes are insoluble in water, whose H2O molecule does not even have a carbon atom; while they are soluble in many organic solvents such as benzene, carbon tetrachloride, chloroform. Furthermore, all liquid alkanes are soluble with each other in all proportions.
Reactivity
Alkanes are very unreactive, as the C-C and C-H bonds are quite strong. Thus, there are only two main reactions for this class of compounds:
- combustion
- halogenation
Combustion
The combustion of alkanes is a reaction of high economic interest, since it is the basis for the production of most of the energy we use every day. Think of petroleum, which is mainly made up of hydrocarbons, namely alkanes, of different lengths. Diesel and petrol, derived from petroleum, are used daily to fuel the internal combustion engines of our means of transport. Furthermore, methane is the molecule that releases the most heat of combustion compared to all subsequent alkanes, given that it has the maximum number of C-H bonds, from which the most energy is drawn. This is why methane is so important and we can use the slogan: ”with methane no complain!”.
To understand more, let’s see in detail the combustion reaction.
A generic combustion reaction of an alkane involves its reaction with oxygen to give carbon dioxide and water.
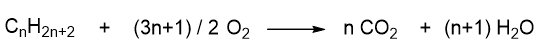
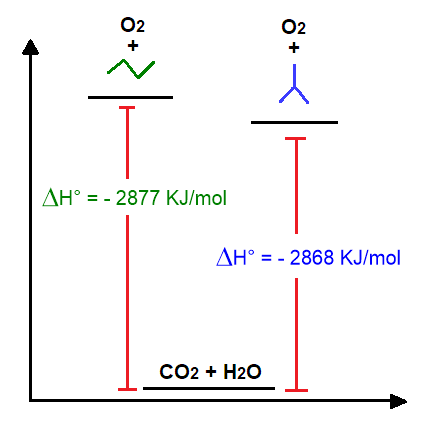
This reaction is exothermic; hence, it develops heat. The stability of the alkane itself can be understood from the heats of their combustions. The higher the heat of reaction developed by the reaction, the more unstable the alkane is. However, only the heats of reaction generated by alkanes that are structural isomers can be directly compared, since these give rise to the same amount of CO2 and H2O during combustion. For example, let’s take the isomers of butane and write their oxidation.

From Figure 15 you can see that linear butane releases more heat than its branched isomer. This implies that normal butane has a higher energy and is therefore more unstable than isobutane See also the diagram below (Figure 16).
The table below shows the heats of reaction for the combustion of the first ten alkanes. What we can notice is that the differences of enthalpies between two consecutive alkanes, which differ for only one CH2, all have a value more or less equal to 658 kJ/mol. This implies that an extra CH2 contributes on average with an extra 658 kJ/mol to the heat of combustion of an alkane. Thus, the heat of combustion of subsequent alkanes can be predicted by simply adding 658 kJ/mol for each additional CH2.

From the table, you can see that methane has the lowest combustion heat of the series, this apparently would seem to be a contradiction to the slogan: “with methane, no complain”, mentioned above. Actually, methane is the one that releases the most heat if you consider that methane has only one carbon. To get an idea, you can divide the heat of combustion by the number of carbon atoms present in the chain and compare the obtained data with the heat of combustion of methane. For example, if we take the enthalpy of combustion of decane (-6829 kJ/mol) and divide it by 10 (number of carbon atoms), we obtain 682.9 kJ/mol, a much lower value than the heat released by methane alone (- 880 kJ/mol).
Halogenation
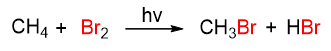
The other reaction of alkanes is halogenation, which consists in the reaction of the alkane with a halogen (F2, Cl2, Br2, I2) to give an alkyl halide. Let’s take the bromination of methane as an example. To take place, the reaction needs a trigger given by a vibration (hv) or by heat; the products, which are formed, are bromomethane and hydrobromic acid
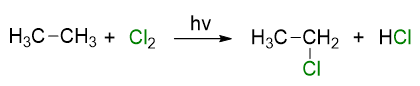
Similarly, if we carry out the chlorination of ethane, we will obtain chloroethane and hydrochloric acid and so on for the halogenations of the subsequent alkanes. The product is always an alkyl halide and hydrogen halide.
The reaction with F2 is too reactive and dangerous, so it has little synthetic value. The reaction with I2, on the other hand, is so slow that it would take years to occur, so this too does not apply. The only really useful halogenations are those with chlorine and bromine.
This lesson

Alkanes
€
1.50
Download as pdf (unchageable) file

Alkanes
€
2.20
Downoald as docx (editable) file




One reply on “Alkanes”
When someone writes an piece of writing he/she maintains the idea of a user in his/her brain that
how a user can understand it. Thus that’s why this post is
perfect. Thanks!