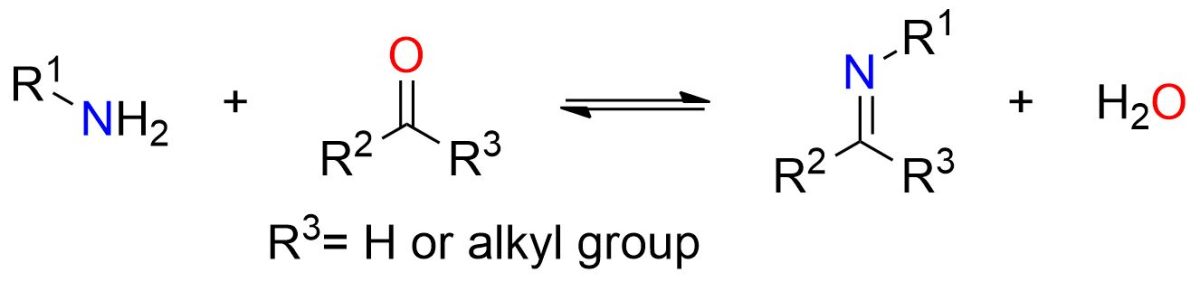Introduction
In this article, we’ll look at the procedures typically used in the laboratory to form imines.
Before we begin, let’s briefly recall what this reaction is.
The imine formation involves the reaction between ketone or aldehyde and a primary amine.
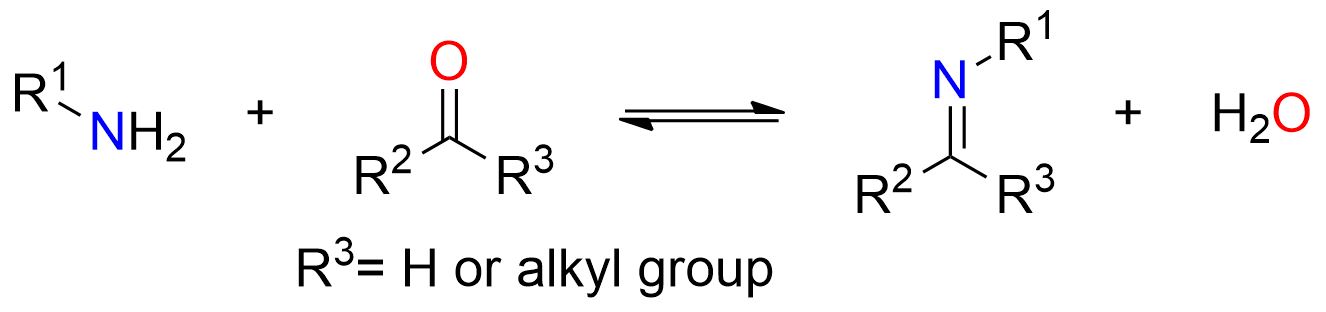
The scheme above illustrates the overall imine formation process. However, this reaction proceeds through multiple reversible stages. Due to the reaction’s reversibility, it’s essential to drive the reaction toward the products by carefully choosing the reaction conditions, as explained below.
General reaction conditions
The formation of an imine is a reversible process; for this reason, it is necessary to shift the equilibrium of the reaction towards the products by using the following strategies:
use of an excess of imine;
removal of the water produced;
acid catalysis, or more rarely, basic catalysis.
These strategies can be used in combination or individually.
For the removal of water from the reaction environment, the Dean-Stark apparatus (see figure below) is commonly used, as it allows the water to be distilled out by taking advantage of the toluene-water azeotrope. However, if this apparatus is not available, other techniques can be employed to eliminate water, such as the use of hygroscopic salts (e.g. Na₂SO₄, MgSO₄, silica gel, etc.) or molecular sieves.
Acid catalysis is typically carried out by adding catalytic amounts of acids such as para-toluenesulfonic acid, hydrochloric acid, acetic acid, etc. The addition of acid serves to make the carbonyl group of the ketone or aldehyde more electrophilic and facilitates the departure of the carbonyl oxygen as water, following protonation.
The reaction is typically catalyzed by acids, but there are examples in the literature of basic catalysis as well. The most commonly used bases in this context are K₂CO₃, NaOH, NaHCO₃, KF, etc.

Typical procedures
After discussing the general characteristics of imine formation, let’s finally see how to carry out the reaction in the laboratory. Below are some procedures found in the literature.
Procedures using a Dean-Stark apparatus
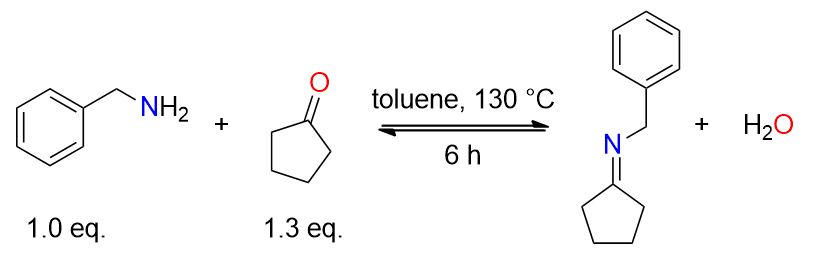
A mixture of cycloheptanone (1.43 mL, 12.1 mmol) and benzylamine (1.02 mL, 9.33 mmol) in 30 mL of toluene was heated at reflux for 6 hours under Dean-Stark conditions for water removal. The resulting residue was concentrated under reduced pressure and used directly for subsequent reactions. [1]
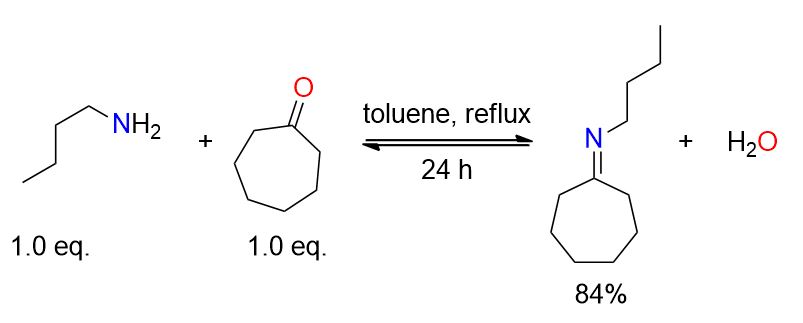
A flask equipped with a Dean-Stark apparatus was loaded with cyclohexanone (10.36 mL, 0.1 mol), 1-butylamine (9.98 mL, 0.1 mol), and 15 mL of toluene. The mixture was heated to reflux and stirred for 24 hours. The solution was then distilled (64°C, 2 mmHg), yielding N-cyclohexylidenebutylamine (12.8 g, 84%) as a colorless oil.[2]
Procedures using molecular sieves
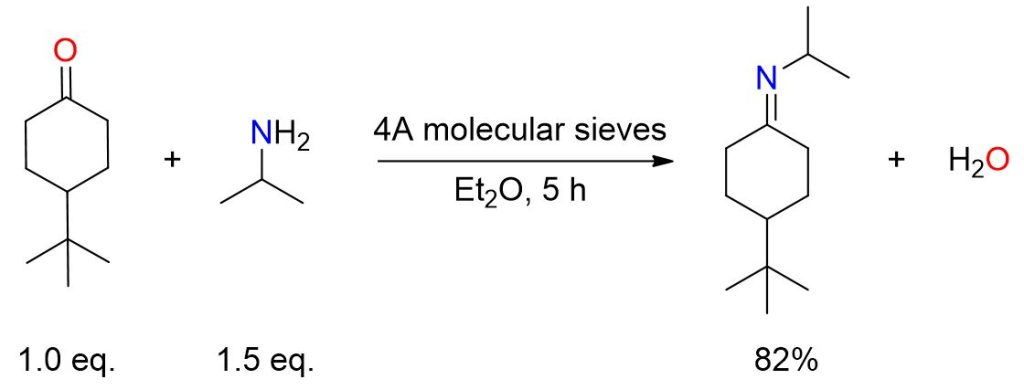
A mixture containing 20 mmol of 4-tert-butylcyclohexanone in 15 mL of anhydrous ether, which included 8 g of 4A molecular sieves, was reacted with 30 mmol of isopropylamine. The solution was stirred for 5 hours, then filtered, and the sieves were rinsed with three 3-mL portions of ether. The combined ether solutions were then concentrated. Finally, bulb-to-bulb distillation of the resulting residue yielded an imine with an 82% yield, boiling point 65-70 °C (0.1 Torr).[3]
Procedures using hygroscopic salts
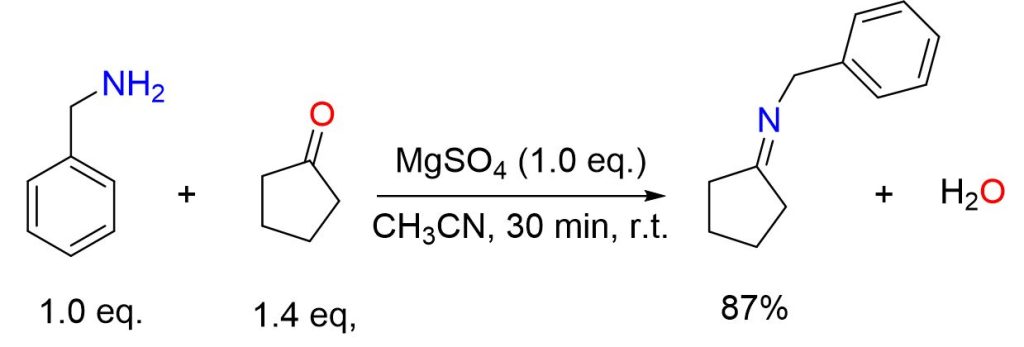
A solution of cyclopentanone (2.47 mL, 28 mmol), benzylamine (2.18 mL, 20 mmol), and MgSO4 (2.4 g, 20 mmol) in CH3CN (30 mL) was stirred at ambient temperature for 30 minutes. Afterward, the MgSO4 was removed by filtration, and the solvent was evaporated, leaving behind a yellow oil (3 g, 87% yield).[4]
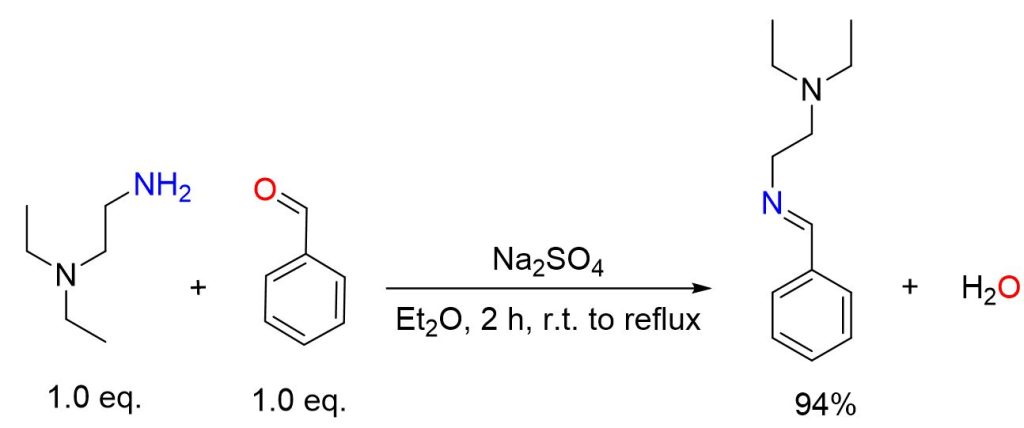
Benzaldehyde (5.30 g, 50.0 mmol) and N,N-diethylethylenediamine (5.81 g, 50.0 mmol), were dissolved in diethyl ether and stirred with sodium sulfate for one hour at room temperature, followed by an additional hour under reflux. The reaction mixture was then filtered, and the solvent was eliminated using a rotary evaporator. The product was subsequently dried under vacuum using an oil pump, (9.60 g, 47.0 mmol, 94% yield).[5]

The (3-chlorophenyl)methanamine (1 eq.) was added to a solution of tert-butyl ester of 4-oxo-piperidine-1-carboxylic acid (1 eq.) in toluene (0.65 M), followed by the addition of SiO₂ (weight ratio 1:1 relative to the 4-oxo-piperidine). This mixture was stirred overnight and subsequently used for further transformations.[6]
Procedures using acids
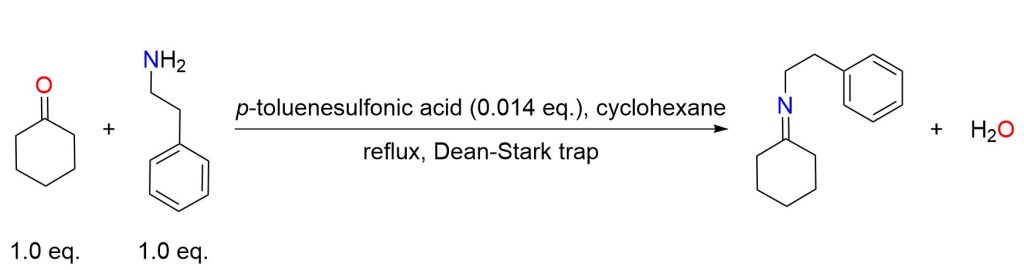
A mixture of cyclohexanone (9.8 g, 0.100 mol), phenylethylamine (12.1 g, 0.100 mol), and p-toluenesulfonic acid (250 mg) in cyclohexane (100 mL) was heated to reflux, with heating maintained until 1 equivalent of water was collected in a Dean-Stark apparatus. The solvent was then removed from the reaction, and the remaining residue was distilled (140 °C at 0.3 mmHg), yielding the intermediate imine as a colorless oil (19 g, 95% yield). [7]
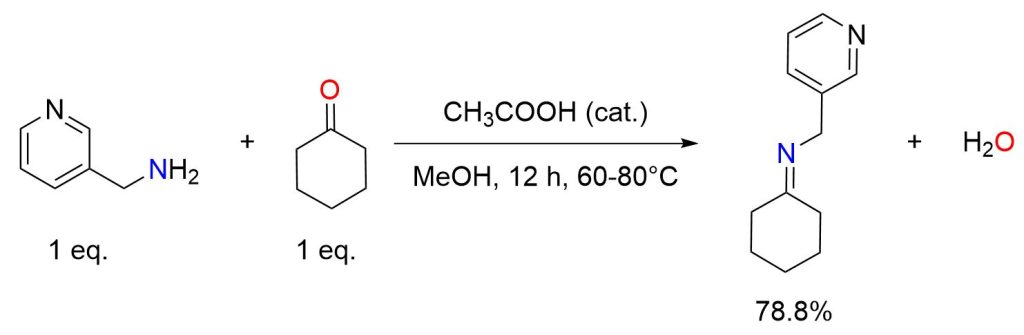
A methanolic solution of 3-aminomethyl pyridine (0.05 mol) was placed in a 250 mL conical flask. To this, an equimolar (0.05 mol) methanolic solution of cyclohexanone was added dropwise, along with a few drops of glacial acetic acid to slightly acidify the reaction mixture. The mixture was stirred for 12–15 hours at 60–80°C using a magnetic stirrer. The reaction’s progress was monitored by thin layer chromatography (TLC). Once the reaction was complete, about half of the solvent was evaporated, and the remaining solution was set aside for crystallization. Recrystallization was then performed using ethanol, to afford the imine in 78.8% yield. [8]
Procedures using bases
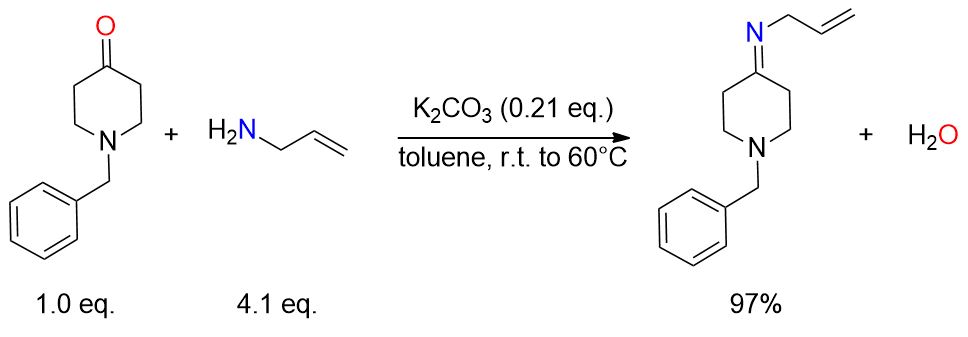
1-Benzyl-4-piperidone (16 g, 84 mmol) and anhydrous K2CO3 (24.3 g, 17.6 mmol) were mixed in toluene (160 mL) under a nitrogen atmosphere. Allylamine (26 mL, 347 mmol, 4 equivalents) was introduced at room temperature. Next, the resulting mixture was stirred overnight at 60 °C, then filtered and evaporated to yield imine (18.78 g, 97%). [9]
References
[1] Monaco, A., Szulc, B. R., Rao, Z. X., Barniol‐Xicota, M., Sehailia, M., Borges, B. M. A., & Hilton, S. T. (2017). Short total synthesis of (±)‐Γ‐Lycorane by a sequential intramolecular acylal cyclisation (IAC) and intramolecular Heck addition reaction. Chemistry a European Journal, 23(20), 4750–4755. https://doi.org/10.1002/chem.201700143
[2] Kusakabe, K., Iso, Y., Tada, Y., Sakagami, M., Morioka, Y., Chomei, N., Shinonome, S., Kawamoto, K., Takenaka, H., Yasui, K., Hamana, H., & Hanasaki, K. (2013). Selective CB2 agonists with anti-pruritic activity: Discovery of potent and orally available bicyclic 2-pyridones. Bioorganic & Medicinal Chemistry, 21(11), 3154–3163. https://doi.org/10.1016/j.bmc.2013.03.030
[3] Fraser, R. R., Banville, J., Akiyama, F., & Chuaqui-Offermanns, N. (1981). 13C shieldings in syn and anti aldimines and ketimines. Canadian Journal of Chemistry, 59(4), 705–709. https://doi.org/10.1139/v81-102.
[4] Baxendale, I. R., Hornung, C., Ley, S. V., De Mata Muñoz Molina, J., & Wikström, A. (2012). Flow microwave technology and microreactors in synthesis. Australian Journal of Chemistry, 66(2), 131. https://doi.org/10.1071/ch12365
[5] Specht, P., Petrillo, A., Becker, J., & Schindler, S. (2021). Aerobic C−H hydroxylation by Copper imine complexes: The Clip‐and‐Cleave Concept – Versatility and Limits. European Journal of Inorganic Chemistry, 2021(20), 1961–1970. https://doi.org/10.1002/ejic.202100185
[6] Rudolph, D. A., Dvorak, C. A., Dvorak, L., Nepomuceno, D., Bonaventure, P., Lovenberg, T. W., & Carruthers, N. I. (2010). Novel tetrahydropyrido[3,2-c]pyrroles as 5-HT7 antagonists. Bioorganic & Medicinal Chemistry Letters, 21(1), 42–44. https://doi.org/10.1016/j.bmcl.2010.11.078.
[7] Gray, N. M., Cheng, B. K., Mick, S. J., Lair, C. M., & Contreras, P. C. (1989). Phencyclidine-like effects of tetrahydroisoquinolines and related compounds. Journal of Medicinal Chemistry, 32(6), 1242–1248. https://doi.org/10.1021/jm00126a016.
[8] Pandey, S., & Srivastava, R. S. (2010). Synthesis and characterization of some heterocyclic schiff bases: potential anticonvulsant agents. Medicinal Chemistry Research, 20(7), 1091–1101. https://doi.org/10.1007/s00044-010-9441-z.
[9] Jenkins, I. D., Lacrampe, F., Ripper, J., Alcaraz, L., Van Le, P., Nikolakopoulos, G., De Almeida Leone, P., White, R. H., & Quinn, R. J. (2008). Synthesis of four novel natural product inspired scaffolds for drug discovery. The Journal of Organic Chemistry, 74(3), 1304–1313. https://doi.org/10.1021/jo802456w.