Introduction
In this article, we will explore the procedures typically employed in the laboratory to carry out the Fischer esterification reaction.
Before we begin, let’s briefly recall what this reaction is.
The Fischer esterification reaction is the formation of an ester from a carboxylic acid and an alcohol, under acidic catalysis.
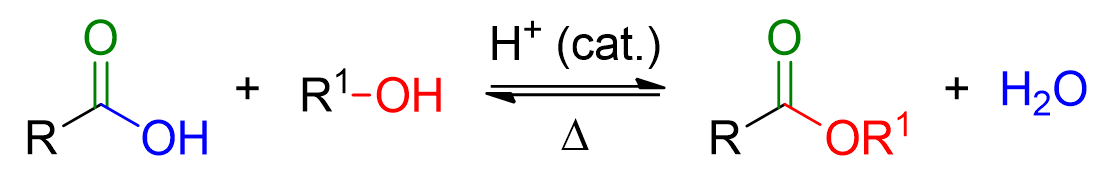
As seen from the diagram above, the Fischer reaction is reversible and requires heat to occur.
If you don’t remember the reaction mechanism, you can find it in our article: ”Formation of esters”
Generic reaction conditions
As mentioned earlier, Fischer esterification is a reversible reaction, so it is necessary to operate under conditions that shift the equilibrium toward the reactants. These conditions are::
use of an excess of alcohol;
elimination of water as it forms.
Often, the alcohol is directly used as a solvent in these reactions. Alternatively, water can be removed from the reaction environment using a toluene/water azeotropic mixture and the Dean-Stark trap.
The figure below shows the typical apparatus used to carry out the reaction using the Dean-Stark trap.

Now let’s look at the typical reagents and temperatures used.
The alcohols used are generally the simpler ones, such as MeOH (methanol), EtOH (ethanol), n-PrOH (n-propanol), n-ButOH (n-butanol), etc.
The acids used as catalysts are usually sulfuric acid (H2SO4), para-toluenesulfonic acid (p-TsOH), and boron trifluoride (BF3).
The reaction is conducted under reflux, so the temperature will be set based on the boiling point of the solvent used.
Typical procedures
After discussing the general characteristics of Fischer esterification, let’s finally see how to carry out the reaction in the laboratory. Below there are some procedures found in the literature.
Procedures with H2SO4 as catalyst
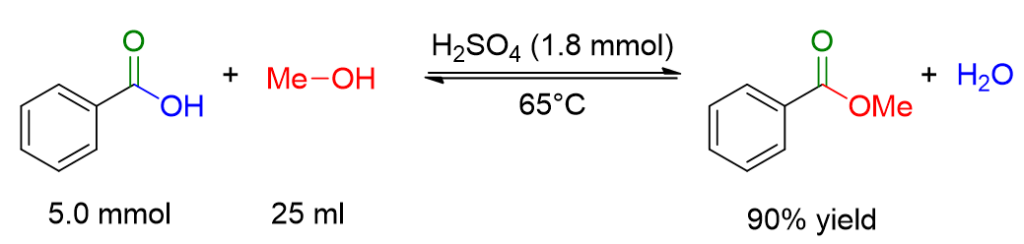
610 mg of benzoic acid were dissolved in 25 ml of methanol. Concentrated sulfuric acid (0.1 ml) was added slowly and cautiously to the reaction mixture. The reaction mixture was stirred at 65°C until the reaction was complete. The solvent was removed under reduced pressure. The residue was extracted with ethyl acetate (50 ml), and the organic phase was washed with a saturated solution of NaHCO3 (2 x 30 ml) and finally with a saturated solution of NaCl. The resulting organic phase was dried over MgSO4 and concentrated under reduced pressure. Methyl benzoate was obtained with a yield of 90%.
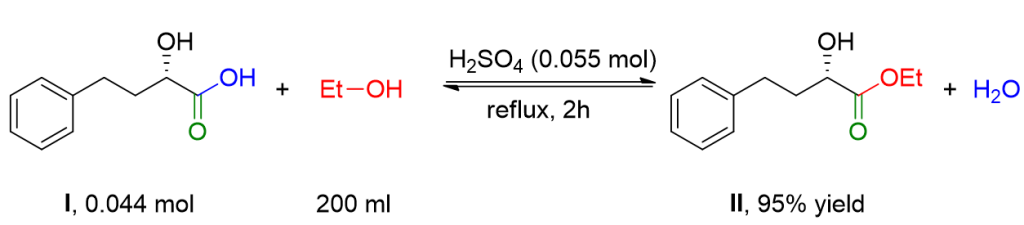
To a solution of hydroxy acid I (8.0 g, 0.044 mol) in anhydrous ethanol (200 ml), concentrated H2SO4 (3 ml) is added. The mixture is refluxed for 2 hours. The solvent is removed under reduced pressure to eliminate excess ethanol, and a mixture of H2O (50 ml) and ethyl acetate (200 ml) is added. The organic phase is washed with an aqueous solution of NaHCO3 and with a saturated solution of NaCl. Finally, it is dried over Na2SO4 and filtered. The solvent is removed using a Rotavapor to obtain ester II as a pale yellow oil (8.9 g, 95% yield).[1]
Procedures with p-TosOH as catalyst
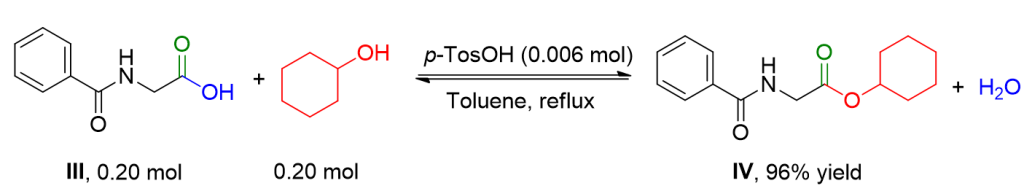
Hippuric acid III (35.8 g, 0.20 mol) and cyclohexanol (20.0 g, 0.20 mol) are refluxed with para-toluenesulfonic acid (1.0 g) in toluene (200 ml). The reaction is conducted with a Dean-Stark trap for water removal until the expected amount of water has formed (typically 30 hours). After cooling the reaction flask, EtOAc (200 ml) is added for dilution. The resulting organic phase is washed twice with water, dried over MgSO4, and filtered. The solvent is removed using a Rotavapor, and the crude product is recrystallized from EtOAc/n-hexane (1:1) to obtain ester IV as a colorless solid (50.3 g, 96% yield).[2]
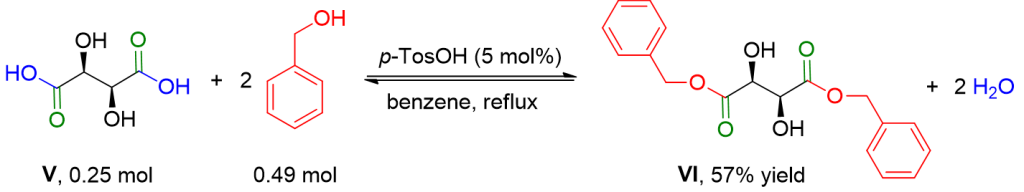
In a round-bottom flask equipped with a Dean-Stark trap with a condenser, connected to a nitrogen cylinder, and a magnetic stirrer, d-tartaric acid V 37.75 g (0.25 mol), benzyl alcohol 53.0 g (195 mol%), para-toluenesulfonic acid monohydrate 2.37 g (5 mol%), and 110 ml of benzene are added. The reaction mixture is refluxed until complete water evolution (20 h) and then brought back to room temperature. Isooctane (100 ml) is added, and the formed precipitate is filtered and redissolved in 300 ml of EtOAc. The organic phase is washed with a saturated aqueous solution of NaHCO3 (2 x 75 ml) and a saturated solution of NaCl (2 x 50 ml), dried over Na2SO4, and filtered. The solvent is removed using a Rotavapor. The crude product is dissolved in toluene (200 ml), and the product is precipitated by adding 200 ml of isooctane. After filtration and drying under high vacuum, compound VI, 46.02 g (57% yield), is obtained as a white solid.[3]
Procedures with BF3 as catalyst
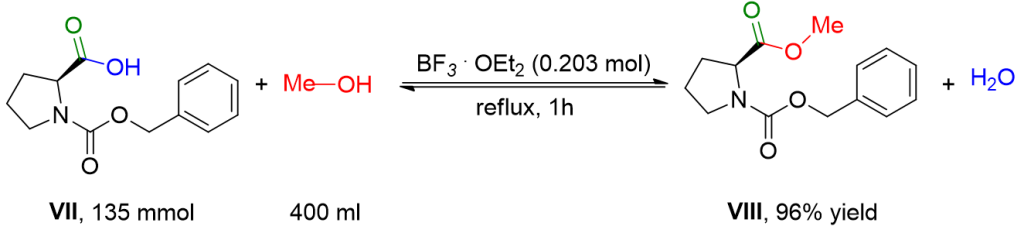
A round-bottom flask, equipped with a condenser and a magnetic stirrer, is charged with 33.7 g (0.135 mol) of N-(benzyloxycarbonyl)-(S)-proline VII and 400 mL of anhydrous methanol under an inert atmosphere. Boron trifluoride (24.6 mL, 28.4 g, 0.2 mol) is added to the mixture with stirring. The resulting solution is then refluxed for 1 hour. The solvent is removed under reduced pressure, and the residue is vigorously shaken with 200 ml of ice-cold water and extracted with EtOAc (3 x 100 mL). The extracts are combined and successively washed with a saturated solution of NaCl, a 1 M aqueous solution of NaHCO3, and a saturated solution of NaCl. The organic phase is dried over Na2SO4 and filtered. After removing the solvent under reduced pressure, the resulting colorless oil is dried by dissolving twice in 100 ml of anhydrous toluene and subsequent removal of the solvent under reduced pressure. Compound VIII is obtained as a colorless oil (34.2 g, 96% yield).[4]
References
[1] Lin, W., He, Z., Jing, Y., Cui, X., Liu, H., & Mi, A. (2001). A practical synthesis of ethyl (R)- and (S)-2-hydroxy-4-phenylbutanoate and d-homophenylalanine ethyl ester hydrochloride from l-malic acid. Tetrahedron-asymmetry, 12(11), 1583–1587. https://doi.org/10.1016/s0957-4166(01)00285-3
[2] Kober, R., Papadopoulos, K., Miltz, W., Enders, D., Steglich, W., Reuter, H., & Puff, H. (1985). Synthesis of diastereo- and enantiomerically pure α-amino-γ-oxo acid esters by reaction of acyliminoacetates with enamines derived from 6-membered ketones. Tetrahedron, 41(9), 1693–1701. https://doi.org/10.1016/s0040-4020(01)96483-x
[3] Bishop, J. K., O’Connell, J. F., & Rapoport, H. (1991). The reaction of thioimides with phosphorus ylides. The Journal of Organic Chemistry, 56(17), 5079–5091. https://doi.org/10.1021/jo00017a017
[4] Corey, E. J., Shibata, S., & Bakshi, R. K. (1988). An efficient and catalytically enantioselective route to (S)-(-)-phenyloxirane. The Journal of Organic Chemistry, 53(12), 2861–2863. https://doi.org/10.1021/jo00247a044
