What does chemistry study?
What does chemistry study?
This is certainly the first question to ask at the beginning of our studies. However, it is also a question that we will be able to understand more deeply over time, as soon as we discover the various fields of chemistry. But now let’s see the classic definition.
Chemistry studies matter and its transformations
since matter is not something static, but is mutable; just think of chemical reactions or even of phase changes from solid to liquid to gas. Moreover, if everything were always the same, we chemists would soon get bored. We would not see the magic of transformations: think for example of fireworks, where the elements enjoy emitting colored lights and brighten up our parties. So keep the term transformation in mind!

What is the matter?
We have just said that chemistry studies matter and its transformation. Therefore, to better understand the definition of chemistry, we just need to define well the concept of matter:
Matter is anything that has mass and volume (takes up space).
It is everything we see and touch. Think of rocks, water, food, people, but also everything we cannot see, think of some colorless gases, such as carbon monoxide for example. This is a gas that I do not wish you to come across as it is lethal and cannot even be smelled. It’s a killer who hides well.
To remember the concept of matter as something with mass and something that takes up space, we can think of a McDonald’s sandwich, whose M can remind us the concept of mass, i.e. weight, and the fact of eating it can make us remember that it will take up quite a lot of space in our stomach! It’s definitely not a healthy kind of matter, but it’s certainly a case in point.

Substances : elements and compounds
We have just understood that matter is a general concept, which includes all the materials around us. However, it is possible to recognize parts of matter that are much more specific and identifiable. An example is the concept of substance. Let’s see its definition.
A substance is a pure matter with a definite chemical composition. Where the term pure refers to the microscopic scale. In other words, the substance is not a mixture of several types of matter, such as garbage or meat, but it is a single type of matter such as water or iron.
A substance consists of simple elements or compounds.
The elements
The definition of element, as it is understood today in the world of chemistry, was given by Dalton for the first time. By measuring the mass of the reagents and products of chemical reactions, he noticed that there was something definite in the ratios with which the substances reacted with each other. So this could only be explained by admitting that matter is not continuous, but is made up of tiny particles: atoms. Hence the definition of element:
- An element is a substance, made up of a single kind of atom. An element is therefore an elementary substance formed by atoms all having the same atomic number (i.e. the same number of protons).ta da un’unica specie di atomi.
Chemical elements are classified in the Periodic Table according to their atomic number and are indicated by specific symbols. The elements are over 100, 90 natural and 21 of synthetic origin. Elementary substances exist in nature in various forms based on their reactivity: isolated atoms (as for noble gases) or discrete units of atoms bonded together, molecules (e.g. O2, Br2, P4, S8), a continuous set of atoms linked with covalent bonds, i.e. polymeric substances (such as boron, silicon, carbon in graphite and diamond) or with metal-type bonds, i.e. metallic substances (e.g. sodium, iron, copper, zinc, etc.). The table (Table 1) below clarifies these cases.
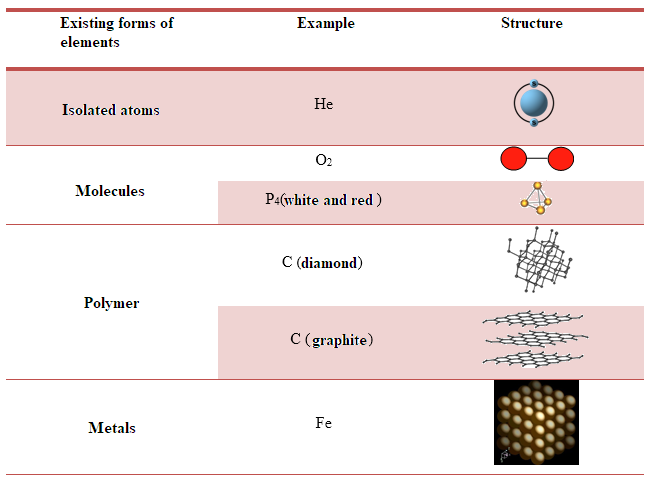
Compounds
Not only elements, but also compounds fall under the classification of substance.
Compounds are substances formed by the combination of two or more elements in a fixed and constant ratio.
This definition derives from Proust’s law of 1799 (law of fixed and constant ratio) according to which: “when two or more elements react, to form a particular compound, they always combine according to fixed and constant mass ratios”. For example, water is made up of two elements: hydrogen and oxygen; however, these elements combine in a way to always respect the ratio of two hydrogen atoms for each oxygen atom. This is briefly synthesized in the molecular formula H2O.
To recap, elements and compounds are both substances. However, elements are made up of only one type of atom, while compounds are a combination of many different atoms. In other words, the elements are those substances identified by a chemical formula whose symbol is found in a single box of the periodic table with at best a number to be added to indicate how many atoms combines: H2, O2, Cl2, P4, S8 etc. While the compounds are represented by chemical formulas, which include symbols deriving from two or more boxes of the periodic table with numbers indicating the ratios with which they combine (see Figure 1). For instance, H2O indicates water, formed both by hydrogen element H and by oxygen element O in ratio 2:1.
Compounds are therefore much more than elements because they derive from the combinations of all the elements of the periodic table with different ratios, therefore the possibilities are potentially endless.
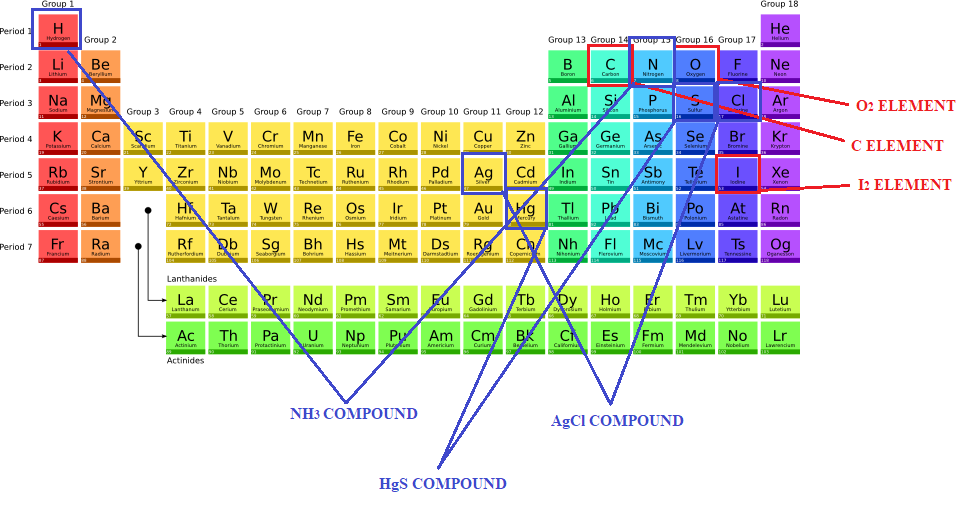
Similar to elements that exist in various forms, compounds can be found as:
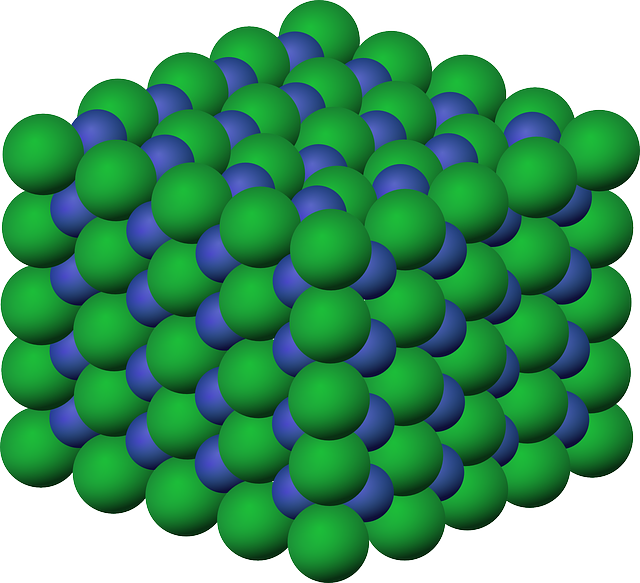
- Ionic compounds: formed by positive and negative ions that interact with each other by electrostatic attraction so that the compound as a whole is electrically neutral. In these compounds it is not possible to identify discrete units, the molecules, because each ion is surrounded by others of opposite charge; however, since the ratios between positive and negative ions are always respected, the ratios are used to define these substances, i.e. the elementary or empirical formula is used. Examples are NaCl (1:1 ratio of sodium ion to chloride) MgCl2, CaCl2 (1:2 ratio of alkaline earth metal ion to chloride ion). All ionic compounds are solid.
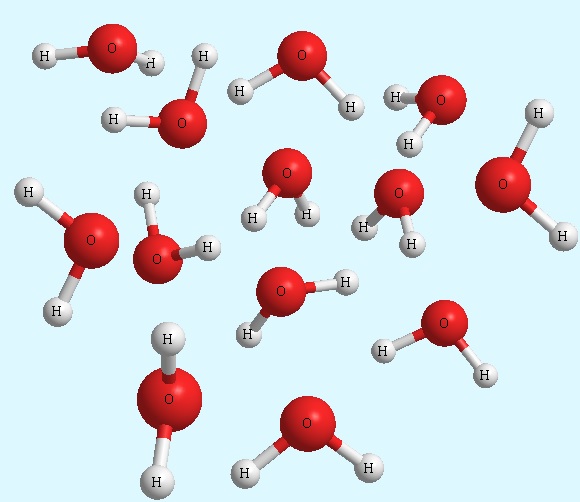
- Molecular compounds: they are substances made up of molecules; where a molecule is a group of atoms linked together in a fixed, distinct and electrically neutral way. They are mostly liquid or gaseous. They are hardly solid, but if they are, they melt at low temperatures. They are expressed by the molecular formula that tells exactly how many atoms there are in each molecule. An example is water expressed with the formula H2O, which states that in a molecule of water there are always two atoms of hydrogen and one of oxygen.
This article

Introduction to Chemistry
€
1.50
Download pdf (unchangeable file)

Introduction to Chemistry
€
2.20
Download docx (changeable file)


