Lewis structures are defined as follows:
Lewis structure is a method to represent atoms, ions and molecules using the octet rule.
Dots are used to indicate the valence electrons of atoms. Two dots (or alternatively a dash) can indicate a lone pair if they are located above one atom or they can indicate a bond if they are shared between two atoms. An isolated electron indicates an unpaired electron (see Figure 1).
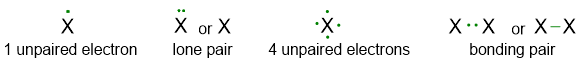
Lewis symbol for single atom
Let us start with single atoms. In this case, the Lewis structure will consist in the symbol of element with the valence electrons around it.
The valence electrons for each element are easily obtainable from the Periodic Table by checking the membership group of that element. In more detail, the valence electrons correspond to the group number for the first two groups in the Periodic Table:
– Group 1 (or IA): the valence electron is 1
– Group 2 (or IIA): the valence electrons are 2
On the other hand, the valence electrons for groups from 13 to 18 can be obtained by subtracting the number 10 to the group number. For instance:
– Group 13 (or III A): the valence electrons are 13 – 10 = 3
– Group 14 (or IV A): the valence electrons are 14 – 10 = 4
– And so on up to the 18th group which possesses 8 valence electrons.
The Lewis symbols always show the same valence electrons for the elements in the same membership group and they reflect the external electronic configuration of those elements.
For example, the Lewis symbol of hydrogen consists of the symbol H with a single unpaired electron (Figure 2), because hydrogen possesses only one electron in its 1s orbital. The same Lewis symbol with 1 unpaired electron will be displayed by the remaining elements of group 1. This because Lewis symbols show only the valence electrons, while electrons from the inner shell are not considered. Therefore, even if the lithium atom has 3 total electrons, the Lewis symbol for Li will consist of one unpaired electron as well, because the two electrons in the 1s orbital are not valence electrons and there is only one valence electron in the outermost 2s orbital. Sodium atom has its valence electron in the 3s orbital and so on for the remaining elements of the first group (see Figure 2 ).

In the following table (Table 1), the Lewis symbols for the main group elements are listed.

Indeed, if I want to write the Lewis symbol for the oxygen, it will be enough to look at the group 16 column in the table above and to exchange X with O; the outcome will be:

The same valence electrons will be used for the elements in the same membership group, e.g. S, Se, Te, Po.
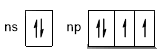
The presence of unpaired or paired electrons in these symbols depends on the distribution of the valence electrons in the outer orbitals. For example, the electronic configuration of group 16 consist of 2 lone pairs and two unpaired electrons, this is because the electronic configuration in this group is ns2+np4 which corresponds to the following scheme:
To understand why electrons arrange in this manner, it is essential to study the electronic configuration of atoms; this is slightly more advanced topic, which requires the knowledge of atomic orbitals. However, it is enough to consider the above table to be able to write the Lewis symbols for the main group elements.
The Lewis model cannot be applied to transition metals, lanthanides and actinides, because these elements have valence electrons also in d orbitals (transition metals) and f orbitals (lanthanides and actinides). The participation of d and f valence electrons confers to those atoms a different behaviour, which can be explained only with more advanced methods involving atomic orbitals.

Would you like to know a trick for calculation of formal charges without memorizing the formula? Find it out at page 6 of the pdf or docx file you can download HERE.
Lewis structures for molecules
The octect rule
The Lewis structures for molecules are based on octet rule. This rule states that
an atom reacts with other atoms forming bonds in order to reach a number of external electrons equal to 8.
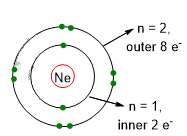
This number of outer electrons gives stability to the atoms and it is also the number possessed by noble gasses (group 18 of Periodic Table). Indeed these elements do not often react and remain usually like single atoms. In the following picture (Figure 4), you can see the atomic representation of one of the noble gases: Neon. Neon has 8 outers electrons in the energetic level n=2. The two electrons in n=1 are considered inner shell electrons with low effect on reactivity.
Nevertheless, there are some exceptions to the octet rule you need to take into account. The following elements are exceptions:
- Hydrogen (H) and helium (He) which require only 2 electrons to be stable.
- Elements from the 3rd period and beyond which can present the so called expanded octet. This term means having around an atom more than 8 electrons, from 10 to 12 electrons. Sulphur (S), phosphorus (P), silicon (Si) are common examples of elements that form an expanded octet. In some compounds, elements like chlorine (Cl), bromine (Br), xenon (Xe), etc. can also show expanded octet.
- Other elements, which can have incomplete octet. It is the case of boron (B) and aluminium (Al), which can employ only 6 electrons to reach stability. A further example is beryllium (Be) which sometimes needs only 4 electrons.
- Transition metals, actinides and lanthanides, which require more advanced methods to understand their reactivity and geometry, as already mentioned.
The 4+2 Rules to build the Lewis Structures
Now, let us see how to build the Lewis structures.
Following the rules below can help you:
- Count the valence electrons for each atom in the molecule and add them up. For this calculation, you need to consider even the negative charges if present. Then divide the obtained sum by two in order to calculate the pair of electrons to work with.
Example) Let us take the anion NO3– and count its valence electrons. N has 5 valence electrons, O has 6, however, since there are 3 atoms of oxygen in the molecule, the total oxygen’s electrons are given by 6×3 = 18 electrons. We have also to consider the negative charge like 1 more electron. So if we add everything up, the result is 24 valence electrons for the molecule.

Let us divide the sum by two to gain the electron pairs to work with.

Note: the valence electrons are the electrons to be placed like lone pairs or bonding pairs around the atoms in the Lewis structure.
- Place the least electronegative atom in the centre of the molecule because this atom is more willing to share its electrons with other atoms. Link this atom to the atoms around by drawing single bonds (two dots or a dash), so that you can have a first draft of the molecular structure.
In the case of our example, the least electronegative atom is the nitrogen (3,04 N vs. 3.44 O). Therefore let us put N in the centre with the oxygen atoms all around. We can link N to O by using a pair of electrons, which can be drawn by a dash (or alternatively two dots). In this way, 3 pairs of electrons have been already employed as bonding pairs, however we still have 9 out of 12 pairs to use.
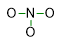
- Calculate the pairs of electrons that are involved in the formation of bonds (bonding pairs). For this purpose, you need to obtain the sum of the total electrons each atom requires in order to gain stability by the octet rule. Therefore, you have to count 8 electrons for all the elements, but those which are exceptions to the octet rule. In this regard, H e He need only 2 electrons; elements such as S o P can expand their octet to 10 or 12 electrons; B e Al can show incomplete octet with 6 electrons. The sum of octets, expanded octets, incomplete octets and duets has to be subtracted to the sum of valence electrons, you have calculated at point [1]. The difference has to be divided by two in order to obtain the bonding pairs.
Back to our example, N needs 8 electrons to be stable and also the 3 oxygen atoms need 8 electrons for each of the atoms; therefore, the sum of octets is given by 4×8 = 32 electrons, which dividing by 2 equals to 16 pairs. The difference between the octet pairs (16) and the valence pairs (12) affords the bonding pairs:

Indeed, the electron pairs, which are involved in bonds are 4. Since in our first draft of the structure we have already settled 3 bonds, only one more bond needs to be placed. Particularly, we have to introduce a double bond between one N-O single bond. So, the structure becomes:
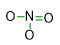
- Place the remaining electron pairs like lone pairs so that each atom of the molecule can respect the octet rule (or the exceptions to the rule).
For NO3– molecole, we have already arranged 4 out of 12 electron pairs, by forming the N-O bonds. As you can see in the picture below, N has already reached 8 electrons by the bonding pairs.
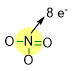
Consequently, we need to place the lone pairs only on the oxygen atoms, in such a way that each oxygen reaches 8 electrons around itself (see picture below).
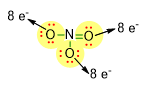
Therefore, 3 lone pairs (highlighted in red) have been placed on the 2 O atoms involved in a single bond and the remaining 2 lone pairs on the oxygen involved in the double bond. Taking into account the negative charge present in the molecular formula of NO3–, we can write the complete Lewis structure as follows:
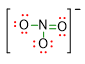
- Check if there are any possible resonance structures. In some of the Lewis structures, it is possible to place multiple bonds in different but equivalent positions. In this case, the structures differ only for the position of the double bond.
In our NO3– molecule, it is possible to shift the double bond by involving a different O atom. In this way, you can gain other two structures, 2 and 3, which are equivalent to the original one, 1.
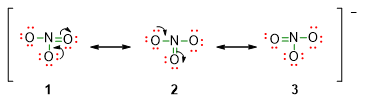
Those structures are all valid and with the same energy. Experiments about resonance structures found that bonds lengths are identical and intermediate between the double bond length and the single bond length.
It is called RESONANCE this combination of structures in which atoms occupy the same positions, while the arrangement of electrons is different. Electrons, which are involved in resonance structures, are considered ‘delocalized’ because the electron density of the double bond is not localized only between two atoms, but it is spread among more atoms in the same molecule.
Now, let’s see the last rule for writing the Lewis structures.
- In some cases there may be uncertainty about more than one possible Lewis structures; in this case, it is good to calculate the so called formal charge, which will help to evaluate the right structure. The formal charge gives an indication about how much electronic charge the atoms have acquired or released when forming bonds. The structure with the lowest formal charges on all atoms is the most stable one.
The formal charge (FC) can be calculated for each atom in the structure as follows:
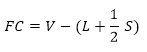
Where:
- V are the valence electrons of the atom;
- L are the lone electrons on the atom;
- S are the shared electrons, namely the bonding electrons.
The Lewis structures have the minimum energy when the formal charges on each non-metals atoms are nearer to zero.
For a neutral molecule, the sum of formal charges must be equal to zero; for an ion, the sum of formal charges must be equal to the charge of the ion.
In the case of our NO3–, the calculation of formal charges would be:
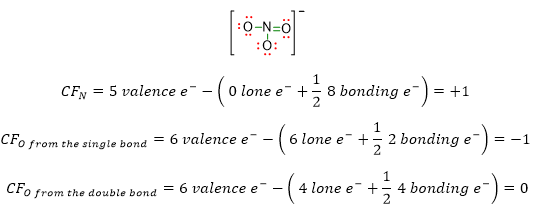
Those formal charges can be written inside the Lewis structure as follows::
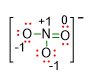
It is to be noted that once you have calculated the formal charge for each atom in the structure, the total formal charge must correspond to the charge of the molecular formula.
In our example, the final structure has total formal charge equal to -1, because, if we sum up the four formal charges of the atoms, the result is 1- ((+1 from N) + 2 (-1 from O) + (0 from O) = -1). This charge fits with that one of the molecular formula NO3–.
To understand better the usefulness of the formal charge calculation, let us take the molecule SO42- like a further example. In this case, we could be unsure about three possible Lewis structures, because we do not know if the sulphur has complete octet, or expanded octet to 10 or to 12 electrons.
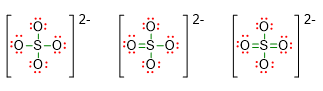
If we write the formal charges for each atoms in these three structures, we will get the following situation:
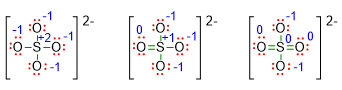
In the first structure, the sulphur has formal charge of +2; such a charge is enough high for a non-metal element, hence we can assume that the first Lewis structure is not stable. In the second structure, S takes on a formal charge of +1, which is lower than the previous one, but it is still a positive charge on a non-metal element. Consequently, the last Lewis structure is the most stable because the sulphur has no charge on it. This last structure is indeed the right one.
This lesson

Lewis Structures
€
1.50
Download as pdf (unchageable) file

Lewis Structures
€
2.20
Download as docx (editable) file