Introduction
How can I make a reagent in water react with a reagent in a water-immiscible organic solvent? At first glance, the reaction would be impossible since the reagents, being in different phases, could not come into contact with each other and therefore react.
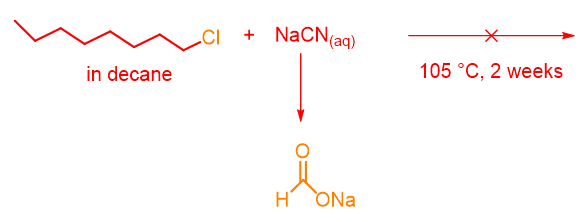
Widely studied in this regard was the reaction between 1-chlorooctane (in decane) and sodium cyanide in water (Scheme 1). The reaction, carried out at 105°C, after two weeks produced only the hydrolysis of cyanide to sodium formate (HCOONa).
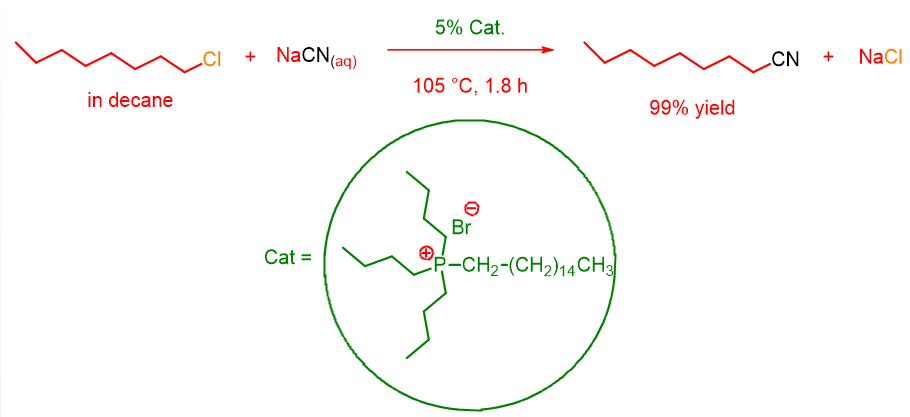
In 1971, the chemist Charles M. Starks[1] demonstrated how this reaction could be carried out in a heterogeneous phase, but with the condition to add a quaternary ammonium or phosphonium salt soluble in the organic phase. In fact, by conducting the reaction with 5% hexadecyltributylphosphonium bromide, 99% yield of cyanooctane was obtained after only 1.8 hours (Scheme 2).
This was one of the first examples of phase transfer catalysis (abbreviation PTC).

Would you like to print this article or save it on your computer and read it offline ? You can download the pdf file here. As alternative you can find also the docx file if you need to change the document.
Definition
Phase transfer catalysis is a technique for carrying out reactions between two or more reactants, which are in two or more different phases, thanks to the use of a phase transfer agent. This agent takes care of transferring a chemical species into the phase where the other reagent is present. However, it is necessary that the transferred species is also activated towards the reaction so that only catalytic amounts of the phase transfer agent can be used.
Therefore, phase transfer catalysis works through a double action: transfer of a reactant and its activation.
Most phase-transfer reactions involve the transfer of an anion, because the organic reactions themselves mostly occur via anions; in this case the transfer is mainly performed by quaternary salts. However, neutral catalysts such as crown ethers, cryptands and polyethylene glycol (PEG) transfer both the anion and the cation into the organic phase. Finally, the first examples of PTC for transferring cations via anionic catalysts have also recently appeared.[2]
In this short article, we will mainly cover the transfer of anions through quaternary ammonium and phosphonium salts; however, there will be references, where appropriate, to the transfer by neutral catalysts.
Anion activation
As mentioned above, the phase transfer catalyst in addition to transferring the anion also activates it. Activation occurs because the bond between the catalyst and the anion has a greater distance than that of the anion and its counter ion. For example, compare the anion-cation distances in NaBr and tetrabutylammonium bromide (Figure 1).
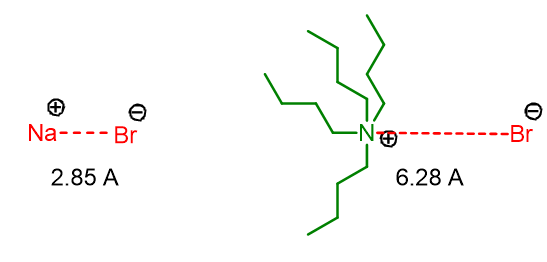
The increased distance in Q+Br- makes the Br- anion more naked and therefore more reactive towards the electrophile in the organic phase.
Another activation mechanism is the fact that the transfer of the anion into the organic phase via the catalyst involves less water of hydration around the anion, making the latter more naked and therefore more reactive.
Mechanisms of phase transfer
Depending on the type of catalyst and reagents used, various mechanisms are possible which we list below.
Simple transfer
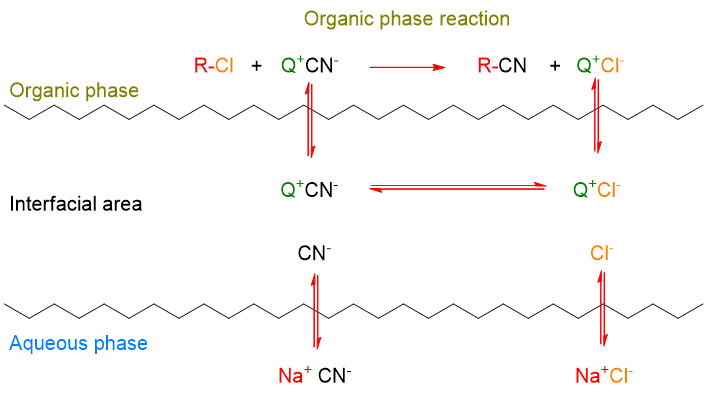
Let’s use again the reaction of cyanide with 1-chlorooctan as an example of a simple transfer. This mechanism occurs according to the following scheme:

As can be seen, the CN– ion is transferred from the aqueous solution to the organic solution by the quaternary ammonium or phosphonium salt (Q+). In organic solution, the CN– ion, which is now even more reactive, reacts with R-Cl to give R-CN. At the same time, the phase transfer catalyst (Q+) binds the counter ion Cl– and brings it into the aqueous phase. Cl– restores the electro-neutrality of the aqueous phase, which, having previously lost the anion CN–, had an excess of positive charges in the form of Na+. Thus, NaCl is formed in the aqueous phase.
This mechanism can easily be remembered by thinking of Q+ as a shuttle service that transports an anion from the aqueous phase to the organic phase, activates it for the reaction and carries the replaced anion back to the aqueous phase.
Very small quaternary salts, such as (CH3)4N+, are soluble only in the aqueous phase and therefore cannot transfer the anion to the organic phase, preventing the reaction. On the other hand, relatively small quaternary salts, i.e. having alkyl chains from 2 to 4 carbon atoms, are capable of being easily partitioned between the organic and aqueous phases according to the simple transfer mechanism. However, the quaternary salts, with chains from 5 to 8 carbon atoms, even if they remain for about 95% in the organic phase, give the highest conversions of the overall process.
The use of even longer alkyl chains on the quaternary salts involves a loss of the catalyst activity: the catalyst will be almost exclusively soluble in the organic phase, losing the ability to partition in the aqueous phase and therefore to carry out the transfer of the anion
Simple transfer through the interface
The second mechanism involves anion exchange at the interface between the two phases. In this case, the quaternary salt does not go into aqueous solution but is limited to carrying out the transfer at the interface (Scheme 4).
Quaternary salts with long alkyl chains such as tetrahexyl and tetraoctyl exhibit this type of mechanism, as they are mainly partitioned in the organic phase (99%), but can interact at the interface. Salts with excessively long chains, completely insoluble in water, however, fail to properly interact at the interface and the reaction is rate-limited by phase transfer.
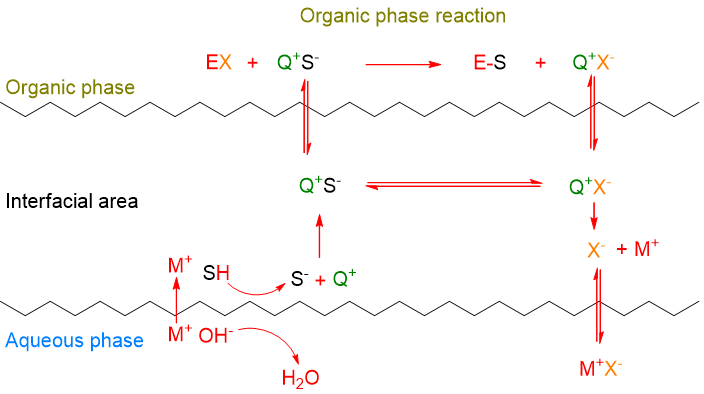
Anion formation at the interface without a catalyst
In the case of some base-catalyzed reactions, the mechanism involves the presence of the base itself at the interface (e.g. OH– in Scheme 5). The base deprotonates an organic acid molecule (SH), transforming it into anion (S–). Only at this point, the phase transfer catalyst (Q+) steps in, transporting the anion from the interface into the internal part of the organic phase, where the reaction between the anion and the electrophile (EX) takes place. The counter ion of the electrophile (X–) is brought to the interface by the catalyst and then goes into the aqueous phase together with the M+ cation coming from the base.
Transfer through reverse micelles
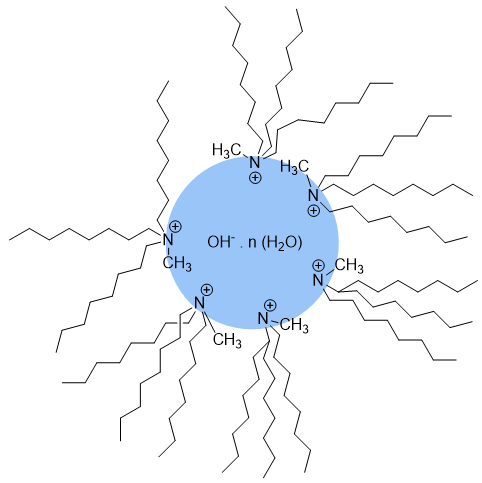
Highly hydrated ions, such as dilute solutions of sodium hydroxide, fluoride and sulfate, make the usual simple transfer or interface mechanism impossible and follow a reverse micelle mechanism. This is because there are too many water molecules strongly bound to the anion, the anion cannot detach from them and is blocked in the aqueous phase. One way to lower the transfer barrier is the formation of reverse micelles (Figure 2), where the quaternary salt forms a micellar structure around the hydrated anion, placing the hydrophobic chains on the outside and the cationic moiety on the inside. The micelle distributes itself into the organic phase, effectively transferring the anion with its water molecules. Consequently, the organic reagent can penetrate into the micelle through the organophilic region of the quaternary salt chains. At this point the reaction can take place.
Third phase formation
When the catalyst is soluble neither in aqueous phase nor in organic phase, it form a third phase. In this case, the mechanism involves the passage of reactants directly in the catalyst phase where their reaction occurs. This type of catalysis is in general even faster than the phase transfer reactions in two phases. Moreover, the insoluble catalyst can be easily removed and recovered at the end of the reaction.
Neutral molecule transfer
Phase transfer catalysts are not only cationic, but can also be neutral molecules such as crown ethers, cryptands, polyethylene glycol (PEG) etc. In this case, the mechanism is different from those seen above. In fact, let us consider the following reaction performed for the first time by F. Molinari in 1974:[3]
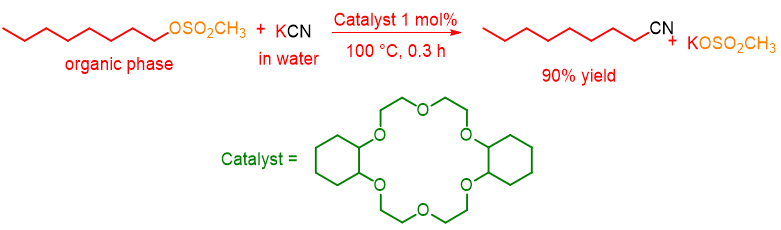
Dicyclohexyl-18-crown-6 catalyzed the substitution reaction of methanesulfonate with CN– in 90% yield in a short time.
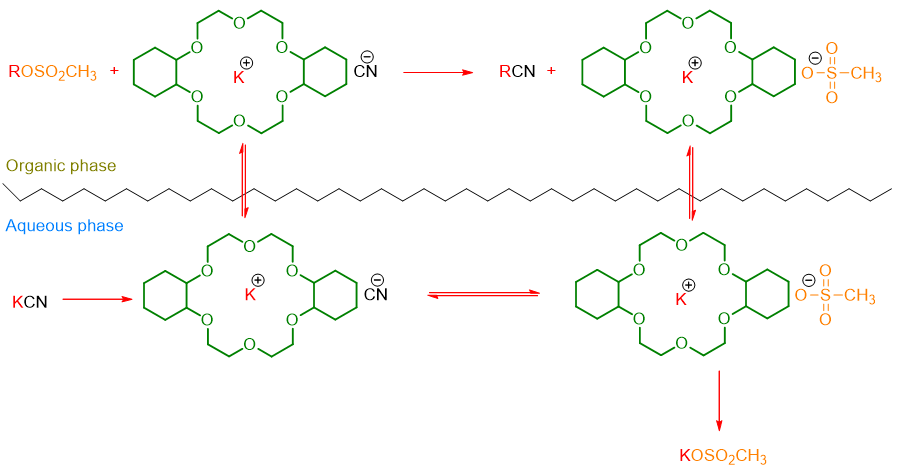
The mechanism of the reaction occurs with the crown ether bringing both the cation and the anion into the organic phase (Scheme 7). In such a situation, the anion is at a great distance from its counter ion and will be able to react even faster in the substitution reaction, being a naked ion.
Speed of phase transfer reaction
In phase transfer reactions the rate of the entire process is actually composed of two rates:
- The rate of the reaction in the organic phase, called the intrinsic rate;
- The speed of phase transfer.
Thus the study of kinetics is complicated due to the presence of two processes (the reaction and the transfer), both with their own speed. In particular, four situations can occur, described by the graph in Figure 3:
- The intrinsic reaction rate is high, but phase transfer is slow (upper left quadrant). In this case we should try to improve the phase transfer;
- Phase transfer is fast, but intrinsic velocity is slow (lower right quadrant). In this case, it is necessary to intervene on the parameters that influence the reaction in the organic phase.
- Both speeds are high (upper right quadrant); it is good here to pay attention that the process does not become uncontrollable;
- Both reactions are slow (lower left quadrant). This is the worst scenario because both processes will need to be tuned up.
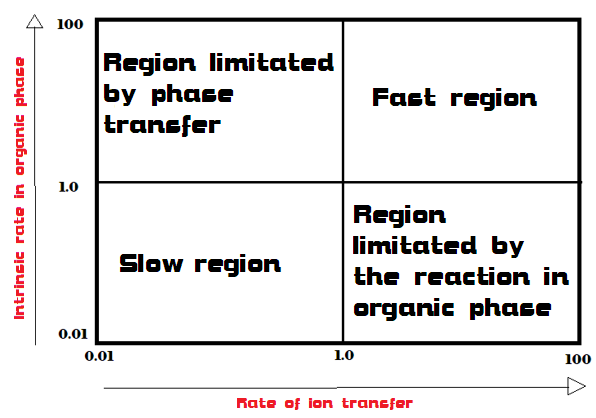
An example of a reaction that occurs in the “fast region” of the graph is the oxidation of organic compounds with permanganate.[4] This reaction is fast both because MnO4– is one of those anions that transfer more easily, and because it is also very reactive in the oxidation of alcohols, alkenes, aldehydes. In addition, an example of a reaction in the “slow region” is the nucleophilic substitution performed with F–. This anion is both difficult to transfer into the organic phase and is a weak nucleophile.
Parameters that influence the speed of phase transfer reactions
To plan a phase transfer reaction, it is good to first get an idea of the rate of the overall reaction. In this regard, the reaction can be thought as divided into two steps: the intrinsic reaction and the phase transfer. To understand whether the reaction in the organic phase will be fast or slow, the reaction in question can be compared with the one performed in the homogeneous phase using the data available in the literature. In fact, it is very probable that if a homogeneous phase reaction is slow, the same will be with phase transfer catalysis.
Regarding the speed of the transfer, one can get an idea of the performance of the anion during the transfer, by consulting the literature on phase transfer catalysis involving the same anion.
Finally, after having studied the literature, it will be possible to take into account the various parameters that can influence the kinetics, bearing in mind that a single parameter can have an effect on phase transfer and an opposite effect on the intrinsic reaction rate. In this case, it is advisable to evaluate the influence of each parameter on each of the two steps. Let’s see below what these parameters are and how to use them.
Catalyst structure
The phase transfer catalyst can influence both the transfer rate and the rate of the intrinsic reaction and this mainly depends on its structure:
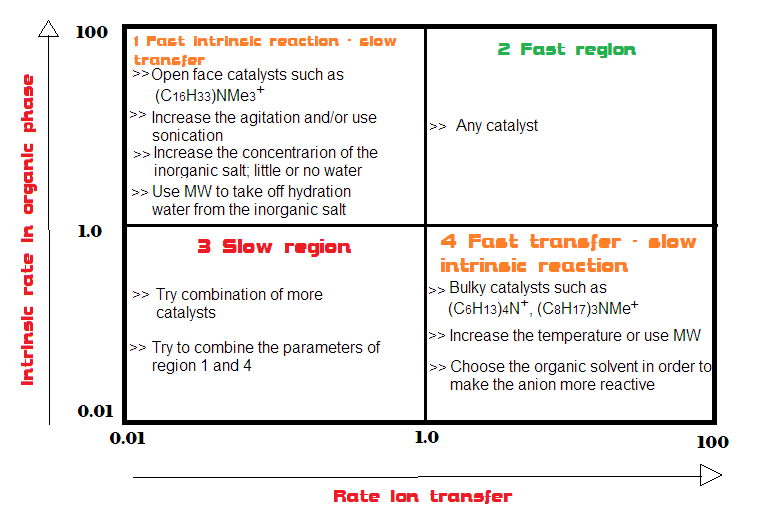
- A quaternary salt with too short alkyl chains (e.g. (CH3)4N+) is not able to partition well in the organic phase and hence greatly limits the speed of phase transfer;
- A quaternary salt with enough long chains (e.g. tetrahexyl- or trioctyl-methylammonium salts) can partition well between the organic phase and the aqueous phase facilitating the phase transfer;
- Very bulky catalysts (e.g. tetrahexyl ammonium salts) are good for intrinsically slow reactions; in fact the anion will be less bound to the catalyst, more naked and therefore more reactive;
- Open-faced catalysts (e.g. hexadecyltrimethyl ammonium), i.e. very accessible on the one hand, are good for increasing phase transfer because they increase the interfacial area of interaction with the anion;
If both reactions are slow, two catalysts can also be used, one that increases phase transfer and the other that activates the anion in the intrinsic reaction.
Conversely, if both reactions are fast, almost any catalyst will be able to carry out the reaction.
Rate of agitation
The rate of agitation is very important in phase transfer reactions because increased agitation generally results in an increase in the anion transfer rate. This is because agitation increases the interfacial contact area between the aqueous and organic phases, allowing the anion to be transferred.
Therefore, in the case of a slow transfer, increasing the stir of the reaction can help; however, this strategy will have no effect on the intrinsic rate of reaction.
Ultrasound can also be used as an agitation tool to enhance anion transfer.
Type and concentration of inorganic salts and quantity of water.
Not all anions transfer quickly. Anions such as MnO4–, ClO4–, I– are known for their high transfer rate. Intermediate speeds result from ions such as Cl–, Br–, NO3–, CN–, NO2–. While the slowest ions to transfer are OH–, F–, ClO–.
In PTC, the use of high concentrations of inorganic salts in the aqueous phase favours phase transfer, because the quantity of the anion will be greater and therefore the catalyst will be able to bind and transfer it more easily. For example, in the case of the substitution reaction of 1-chlorooctane with NaCN, high concentrations of NaCN or even its use as a solid favour the formation of the Q+CN– bond and make the bond between Q+Cl–, which involves the leaving group, less competitive in the aqueous phase.
Therefore, the use of saturated concentrations of the anion are advisable to increase the phase transfer. This can also be done by decreasing the amount of water present. Sometimes a few drops of water are sufficient for the transfer or even the hydration water of the inorganic salt is enough. Less water also helps to have less hydrated anion, which are more difficult to transfer due to the strong bond with water molecules.
Catalysts such as crown ethers and PEGs can carry out the transfer of the inorganic compound even starting from the solid in the absence of water.
In addition, the counterion of the anion to be transferred plays an important role in the solubility of the anion itself. For example, the use of KCN instead of NaCN favours the solubility of the salt and therefore increases the concentration of CN- ions in the aqueous phase.
Temperature
Temperature generally accelerates reactions and so does in PTC, however the catalyst may decompose at certain temperatures. For example, quaternary ammonium salts are known to resist up to 120-150°C, but they also decompose at 50-70°C in alkaline solutions of KOH and NaOH. Crown ethers or polyethylene glycols are resistant under basic conditions, but are sensitive under acidic ones.
The use of microwaves in PTC has generally resulted in an increase in the speed of the overall process. The reason for this effect is due to the ability of the microwave to interact especially with water, removing the hydration molecules from the anion, which become more reactive.
Organic Solvent
Unlike other reactions, PTC offers the possibility of not using any organic solvent in case the reactants are liquid; otherwise, a solvent is necessary if the organic substances are solid at the operating temperature.
However, the organic solvent has an influence above all on the intrinsic rate, because if it is polar but not protic it can activate the anion towards the reaction. The most used solvents in PTC are dichloromethane, toluene, hexane, heptane. Dichloromethane is a solvent polar enough to solubilize most quaternary salt catalysts and to speed both phase transfer and the intrinsic reaction.
A solvent in which the catalyst is insoluble can also be chosen to favour the formation of a third phase.
Summary Table of the effects on speed in PTC
The following table summarizes the parameters to change in order to improve the rate in PTC reactions according to the starting situation (reaction limited by transfer, fast overall reaction, slow overall reaction and reaction limited by reaction in organic phase).
This lesson

PTC
€
2.00
Download as pdf (unchageable) file

PTC
€
4.00
Downoald as docx (editable) file
Riferimenti
1) Starks, C. M. (1971). Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. Journal of the American Chemical Society, 93(1), 195–199. https://doi.org/10.1021/ja00730a033;
2) Rauniyar, V., Lackner, A. D., Hamilton, G. S., & Toste, F. D. (2011). Asymmetric Electrophilic Fluorination Using an Anionic Chiral Phase-Transfer Catalyst. Science, 334(6063), 1681–1684. https://doi.org/10.1126/science.1213918;
3) Landini, D., Montanari, F., & Pirisi, F. M. (1974). Crown ethers as phase-transfer catalysts in two-phase reactions. Journal of the Chemical Society, 21, 879. https://doi.org/10.1039/c39740000879;
4) Lee, D. G. (1982). Phase Transfer Assisted Permanganate Oxidations. In Organic chemistry (pp. 147–206). Elsevier BV. https://doi.org/10.1016/b978-0-12-697253-5.50007-9;
5) Starks, C. M., Liotta, C. L., & Halpern, M. E. (1994). Phase-Transfer Catalysis: Fundamentals, Applications, and Industrial Perspectives. http://ci.nii.ac.jp/ncid/BA23074393

2 replies on “Phase transfer catalysis (PTC)”
Excellent
Thank you