Introduction
In this article, we talk about Syngas. We are going to learn what syngas is, how it is synthesized in industry and some of its transformations.
Definition
Syngas, also known as synthesis gas, is mainly composed of CO, H2 but also N2, CH4, CO2.
Anyway, we can use the word syngas if the mixture contains at least CO and H2.
Production
Syngas is produced for:
- 54% from coal
- 30% from oil
- 3% from natural gas
In theory, it can be produced from any carbon source with a variable percentage of C and H. In particular, the hydrocarbon is reacted with an oxygen source; depending on the oxygen source used, different processes are possible:
- Steam reforming where water is used as a source of oxygen;
- Partial oxidation using oxygen;
- Dry reforming using carbon dioxide;
- Combination of steam reforming and oxidation, using water and oxygen.
Let’s see these 4 processes one by one.

Would you like to read this article offline? Would you like to print it? Or would you like to modify this article for your purpose? You have a chance to download the pdf or docx file here.
Steam reforming
The term steam reforming indicates a reaction in which a hydrocarbon reacts with water in the presence of a catalyst.
Since the reaction takes place in the presence of a catalyst, it is necessary to eliminate any traces of sulfur compounds in the starting material used for the synthesis. After desulphurization, steam reforming can be carried out. The steps are summarized in the diagram below taking methane as starting material.

If the hydrocarbon is methane, the steam reforming reaction is as follows:

The reaction between methane and water vapour is endothermic and reversible.
Instead of methane, propane or naphtha can also be used in steam reforming. As can be seen below, propane gives the steam reforming reaction with higher CO/H2 ratios than methane, especially if the amount of steam is increased (second reaction below):
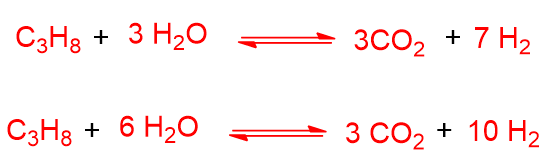
If we take the steam reforming of methane as an example, an undesirable reaction that could occur during this process is the decomposition of methane to form coke. This is a carbon substance with a high C/H ratio, which could block the conversion in the reactor by clogging the pipes where the catalyst flows. The decomposition of methane takes place according to:

Another unwanted reaction is the disproportionation of CO, called the Boudouard reaction, which yields coke too.

To minimize the formation of coke, the pressure of the methane can be lowered and an excess of water compared to the methane (3.5/1 H2O/CH4) is added to cause the following reactions:
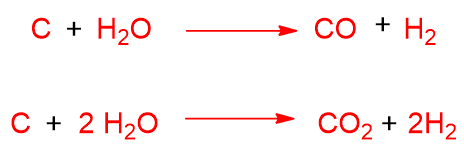
From the above reaction, it can be seen that the CO2/CO ratio increases as the amount of water increases. Furthermore, if the amount of water is increased, the following reaction can also take place:

This is known as water-gas or shift reaction, a slightly exothermic reaction that transforms CO into CO2 through the use of H2O and iron oxides as a catalyst. This reaction increases the H2/CO ratio of the syngas, thus allowing the syngas to be modified according to the relative amounts of the two gases needed. For example if you are synthesizing ammonia then you will need a high H2/CO ratio. The reaction can also reverse if CO2 is added and give CO again, hence the name shift reaction.
In order to have more compact reactors, the steam reforming industries opt for reactors that operate at high pressures, despite this choice thermodynamically disadvantages the conversion of the hydrocarbon into syngas. In fact, the more the pressure increases, the more, according to Le Châtelier’s principle, the equilibrium of the steam reforming reaction moves towards the reagents, where the number of moles involved is lower, so that it is possible to minimize the pressure increase. However, to make this technical requirement of industry possible, the temperature must be increased to shift the equilibrium back towards the products, since the steam reforming reaction is endothermic.
In conclusion, to obtain a good result from steam reforming, one operates in this regime: high pressure, high temperature, high H2O/CH4 ratios or more generally H2O/hydrocarbon.
Dry reforming
This reforming is very similar to the previous one, but instead of using water as oxidation source, it employs carbon dioxide. See the following reaction for methane:

Partial oxidation
Syngas can also be produced through partial oxidation starting from various substrates such as coal, methane, naphtha. This method is chosen when the feedstock consists of liquid hydrocarbons.
After having operated the partial oxidation, the soot must be removed and then the products must be desulphurized before being able to have the syngas. See the diagram below, which summarizes the various stages of the process.

Partial oxidation reactions are exothermic and irreversible, although less exothermic than the complete oxidation of hydrocarbons.
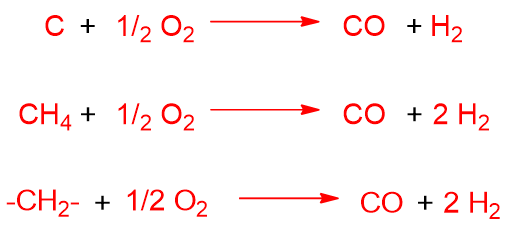
Also in this process, undesired reactions can take place such as complete oxidation, the formation of CO2 from CO and the formation of water from H2 and O2.
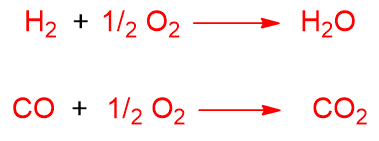
The heat produced by partial oxidation can actually promote the steam reforming and dry reforming reactions, seen earlier. These reactions do nothing but enrich the synthesis gas. Let’s look at them again in the case of methane:
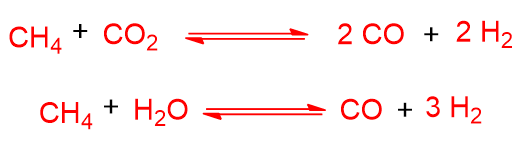
In the case of partial oxidation, pure O2 must be used, this means that the plant must produce pure oxygen (through techniques such as air liquefaction, pressure swing adsorption or temperature swing adsorption) or it must buy it from third-party suppliers.
Combination of steam reforming and partial oxidation
Steam reforming can be combined with partial oxidation, adding O2 to the steam. This combination offers the advantage of using an exothermic reaction (partial oxidation) together with an endothermic one (steam reforming) and therefore of operating without external heating: an autothermal process.
Gassification
Gasification is operated with starting material such as coal. After gasification the particulate must be removed before the syngas can be isolated. Here is a diagram of the processes involved:

This process differs from steam reforming because its main purpose is to transform coal into gas and not to produce syngas. Therefore, steam reforming is done specifically to produce syngas to be used for chemical purposes, while gasification is done to obtain gas from which energy can be obtained.
The syngas produced in this way contains not only CO and H2 but also other components.
Coal is reacted with H2O and O2 or air. If we schematize coal with C, the endothermic reactions that take place are as follows:
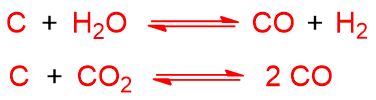
While exothermic reactions are:
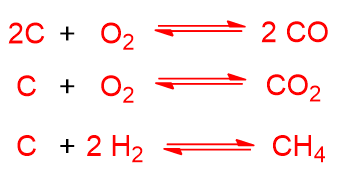
The following exothermic reactions can also occur but in a homogeneous phase:
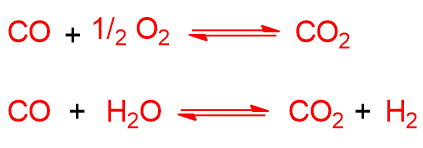
The presence of CO2 in the reactor can be explained by these last reactions. Obviously, given the complex raw material, other reactions are possible, however the ones listed are the main ones.
During gasification, various products are formed which can be divided into two macro-categories: a solid residue and a gaseous one.
In fact, in the coal heating phase, the thermal decomposition of the organic molecules occurs with the formation of radicals. Some of these radicals can condense or polymerize giving rise to the solid residue of the gasification process known as char. The more volatile compounds, on the other hand, are part of the gaseous phase and can be syngas, formed with the reactions described above, or volatile aromatic organic molecules.
The gaseous phase therefore contains H2O, CO2, CO, H2, CH4 and volatile aromatic compounds.
If the gasification is carried out in an autothermal regime, i.e. without the application of external heat, then oxygen is added to the feed. The consequence of the greater presence of oxygen is the formation of greater quantities of CO and CO2 compared to H2 and CH4.
Transformation of syngas
Syngas is used as:
- chemical substrate for the synthesis of NH3, hydrocarbons (Fisher-Tropsch process), methanol, aldehydes and carboxylic acids.
- energy vector, it is transformed into petrol in countries that cannot work with mixtures of naphtha and diesel
Synthesis of ammonia
Ammonia from syngas is synthesized using the Haber process, according to the following reaction:

The reaction takes place only in the presence of the iron-based catalyst, otherwise the stability of the nitrogen is so high that the reaction would not occur.
This reaction is exothermic, therefore it is favoured at low temperatures. Moreover, if the pressure is high, this favours the shift of the equilibrium to the right.
Natural gas is used for the production of ammonia because it is the one that produces syngas with the highest concentration of hydrogen.
The plant for the production of ammonia from natural gas includes the following stages:
- Desulfurization of natural gas;
- First steam reforming of natural gas;
- Second steam reforming with the addition of air and more methane; the addition of air allows the formation of CO2 from O2 and CO and allows the shift reaction to be carried out. This step is autothermal because due to the presence of O2, partial combustion also takes place, which is exothermic. The heat created is used for the endothermic reactions of reforming;
- Shift reaction operated at low temperature and in the presence of a copper-based catalyst;
- Methanation of residual CO and CO2 to transform them into methane, which is not harmful for the Haber reaction; while the carbon oxides must be eliminated because otherwise they deactivate the catalyst. Methanation is the reaction between CO or CO2 and H2 to give CH4 and H2O;
- Condenser of impurities;
- Gas sent (H2 and N2) to the reactor for the production of ammonia.
So ammonia is produced from steam reforming by adding air, which contains N2, to the mixture of CO and H2, obtained from the first steam reforming.
The air, however, which also contains O2, allows CO to be eliminated according to the reaction:

The CO2 can then be removed with ethanolamine and stripping.
Synthesis of methanol
Syngas can be used to produce methanol according to the reactions:
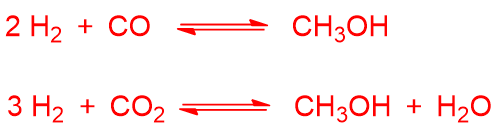
Carbon dioxide can derive from the coupling reaction, i.e. the reaction between carbon monoxide and water if present (also called water-gas reaction or shift reaction):

All of these reactions are exothermic. However, the conversion of CO to methanol is favoured at low temperatures and high pressures, while the conversion of carbon dioxide to methanol is favoured at high temperatures, due to the water-gas reaction involved. To promote the methanol formation, high pressure should then be used which favours both conversions.
To date, however, copper-based catalysts capable of operating at low pressures and low temperatures have been discovered. However, for the catalysts to work, they need syngas free from sulfur contamination.
The catalyst must also allow high selectivity towards methanol formation since other products would be possible, such as methane, ethane, propane and ethanol.
To this end the best catalyst in use today is KATALCO 55-1, which consists of zinc oxide, copper and alumina, where copper is the active catalyst. Selectivity exceeds 99%.
The syngas, which is used for the formation of methanol, is that coming from the steam reforming of naphtha, because high H2/CO ratios are necessary to obtain methanol. The favourable ratio is at least 2/1 H2/CO. If syngas from natural gas were used, there would be a higher H2/CO ratio and this would not be convenient for the plant, given that the excess of hydrogen would then have to be treated. If the syngas, on the other hand, has a lower value of 2/1 H2/CO, more by-products would be formed such as longer chain alcohols. Therefore, the ideal ratio is 2/1.
If such a syngas is not available, one can:
- Add CO to the process, in case of syngas too rich in hydrogen;
- Add a second stem reforming with oxygen feed after the water steam reforming, to increase the CO content.
- Eliminate excess hydrogen after the reaction
Synthesis of Fischer-Tropsch
This process converts the syngas into hydrocarbons with chain lengths in the range of C2-C19.
Typical reactions to give alkanes or alkenes are:

However, these reactions are accompanied by possible side reactions such as:
- the shift reaction, which however can be used to lower the amount of CO, which may not be compatible with certain catalysts for the Fischer-Tropsch reaction (see scheme 20);
- formation of alcohols according to:

from a thermodynamic point of view, the formation of alcohols is favored over alkanes.
- formation of coke at high temperatures; this reaction is irreversible and unwanted because it compromises the reactor. To avoid it, it is necessary to keep the temperatures not too high.

The chain length obtained in the Fischer-Tropsch process depends on the CO/H2 ratio, the temperature and the catalyst used. At higher temperatures, short chains are favored. As regards, however, the influence of the catalyst, typically to have:
- long chains Co catalysts are used. These catalysts are active between 200-240 °C with ratios of syngas H2/CO 2.15/1. Higher ratios of hydrogen to carbon monoxide are needed because this catalyst does not undergo the shift reaction, which consumes CO and produces hydrogen. Since cobalt is more expensive, we try to increase its surface area using supports such as Al2O3, silica etc. This catalyst, being very active, most likely leads to the formation of waxes. Hydrocracking is used to bring the waxes to a lower molecular weight (at high temperatures and in the presence of hydrogen, the chains are reduced in size).
- short chains Fe catalysts are used. This catalyst is active between 300-350 °C with ratios of syngas H2/CO 1.7/1. Compared to Co it is superior for CO-rich syngas since it also catalyzes the shift reaction. Fe catalysts mostly return short linear chains both alkanes and olefins; to increase the yield somewhat in longer chains, an alkaline additive, such as K2O, can be added.
Therefore, the most used catalysts in the Fischer-Tropsch synthesis are cobalt, iron, ruthenium because they have half-filled d orbitals and are able to propagate the chain well. Other catalysts such as nickel, although very reactive, mainly affords methane, which is why it is not used.
It is advisable to avoid carbon formation during the reaction because this would deactivate the catalyst especially those based on iron, while Co is more sensitive to sulfur compounds which may be present in the raw material used to make the syngas.
This lesson

Syngas
€
1.50
Download as pdf (unchageable) file

Syngas
€
2.50
Downoald as docx (editable) file

