Introduction
In this article, we’ll look at the procedures typically used in the laboratory to carry out the saponification reaction.
Before we begin, let’s briefly recall what this reaction is.
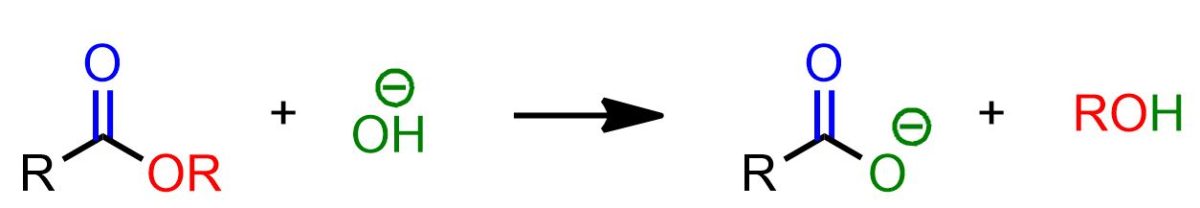
The saponification reaction is the hydrolysis of an ester using hydroxides.
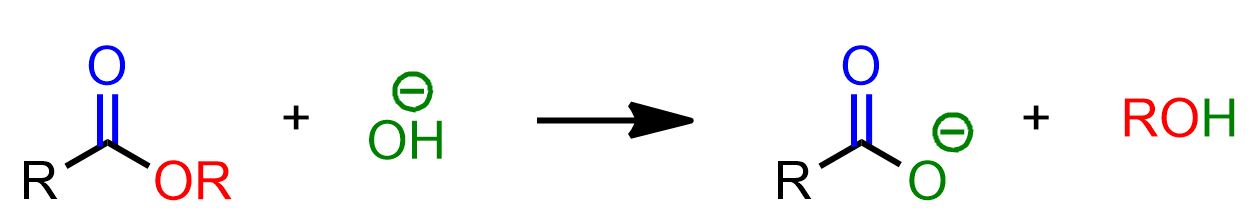
The scheme above illustrates the saponification reaction as a whole. However, this reaction occurs in multiple stages, with only the final one being irreversible. In this last stage, an acid-base exchange occurs between the carboxylic acid and the alkoxide ion. This final step is the driving force behind the entire reaction, which would otherwise be reversible.
General reaction conditions
The typical conditions for the basic hydrolysis of esters are:
use of an excess of hydroxide;
room temperature or reflux .
acidic step after saponification.
So the reaction can be carried out at room temperature or under reflux, depending on the ease of hydrolysis of that particular ester.
The most typical solvents used in the reaction are alcoholic solvents, such as MeOH or EtOH, which are capable of solubilizing the hydroxides. Water is often added as well to facilitate this solubilization.
The most commonly used hydroxides are NaOH and KOH.
A variation of the reaction involves enzymatic catalysis using lipases in water.
All saponification procedures require a final acidic step, typically achieved by adding HCl to the aqueous solution after extraction. This step is necessary because, after saponification, the carboxylic acid exists in its deprotonated form (carboxylate), thus requiring an acid to protonate it.
Typical procedures
After discussing the general characteristics of saponification, let’s finally see how to carry out the reaction in the laboratory. Below are some procedures found in the literature.
Procedures using NaOH as the hydroxide
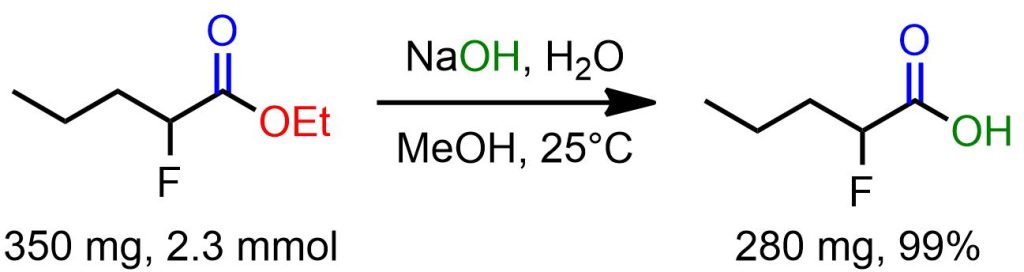
The ethyl 2-fluoropentanoate (350 mg, 2.3 mmol) was dissolved in a mixture of methanol (5 ml) and aqueous solution of 1 M NaOH (5 ml). The resulting mixture was stirred for 5 hours at room temperature. Subsequently, HCl (1 M) was added until the completion of the acidic reaction. The mixture was then extracted with ethyl acetate (3 x 50 ml). The combined organic phases were washed with water and brine, dried over Na2SO4, filtered, and evaporated under reduced pressure to yield 250 mg of 2-fluoropentanoic acid (99% yield) as a colorless volatile oil. [1]
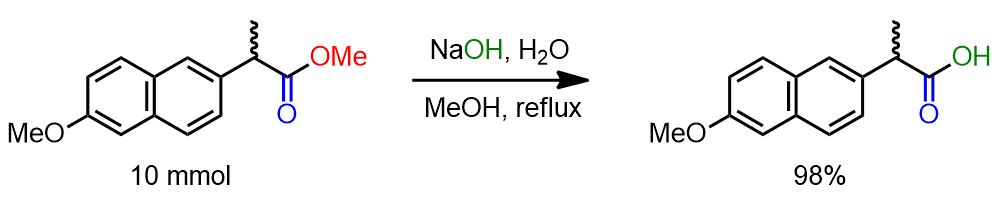
The ester was dissolved in a 30% aqueous solution of NaOH (15 ml) in MeOH (50 ml) and refluxed under stirring for 4 hours. The reaction mixture was poured into water and extracted with ethyl ether (2 x 50 ml). The aqueous phase was acidified with concentrated HCl and extracted with ethyl ether (3 x 80 ml). The organic phase was washed with water, dried over Na2SO4, and filtered. The solvent was removed using a Rotavapor to obtain the carboxylic acid (98% yield).[2]
Procedures using KOH as the hydroxide
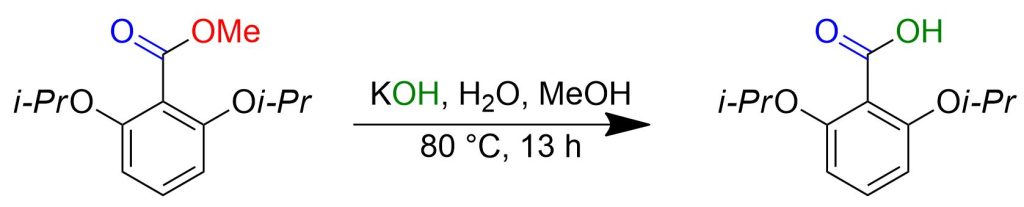
Potassium hydroxide (20 g, 0.36 mol) was dissolved in a mixture of methanol (90 mL) and water (10 mL) in a 9:1 ratio. To a 20% weight solution of KOH, methyl 2,6-diisopropoxybenzoate (8.3 g, 0.033 mol) was added, and the mixture was heated to 80°C (oil bath temperature). After stirring for 13 hours at 80°C, the mixture was cooled to room temperature, poured into 1 N aqueous HCl, and extracted twice with ether. The organic phases were concentrated under reduced pressure, and the crude solid was washed with hexane and dried to obtain 2,6-diisopropoxybenzoic acid (7.7 g, 0.032 mol) as a white solid with a yield of 98%.[3]
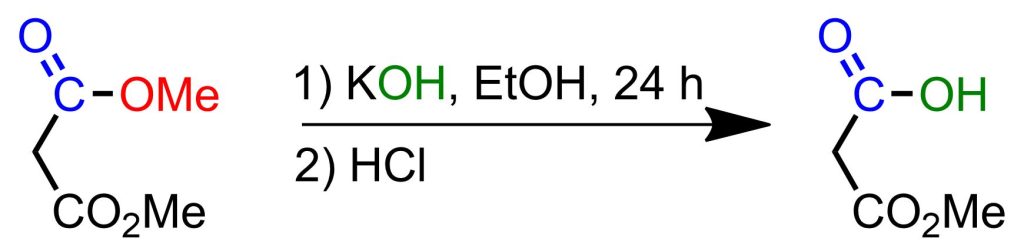
1 mole (132 g) of dimethyl ester of malonic acid is added dropwise to 500 ml of absolute ethanol, to a solution of 1 mole of KOH (56 g) in 500 ml of absolute ethanol. After 24 hours, the obtained potassium salt is filtered, washed with a small amount of ether, and dried under vacuum. Dropwise addition is done to a solution cooled with an ice bath containing 1 mole of potassium salt and 100 ml of ice-cold water, with 2 moles of concentrated HCl. The mixture is extracted 3-4 times with 100 ml of diethyl ether each time, dried over sodium sulfate, and the oily residue is distilled under vacuum. A colorless liquid is obtained with almost quantitative yield, boiling point 84°C/0.18 Torr (literature: 168°C/12 Torr).[4]
Procedures using LiOH as the hydroxide
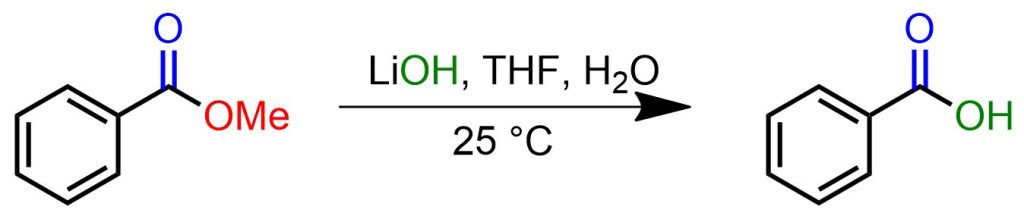
A mixture of methyl benzoate (544 mg, 4 mmol), LiOH (960 mg, 40 mmol), and water (4 ml) in THF (20 ml) was stirred at room temperature until the completion of the reaction (monitored via TLC). The reaction mixture was then acidified with 1 N HCl followed by extraction with DCM (2 × 60 ml). The combined organic phases were dried over anhydrous MgSO4 and concentrated under vacuum to obtain pure benzoic acid (88%).[5]
Procedures with lipase
Example of enzymatic kinetic resolution.
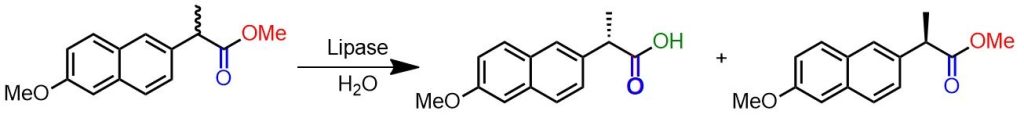
Finely powdered racemic methyl ester of naproxen (244 mg, 1.0 mmol), mercaptoethanol (1 μmol), and polyvinyl alcohol (10 mg) are added to crude lipase from C. cylindracea (Sigma L1754 Type VII, 600 μg protein) in a 0.2 M phosphate buffer at pH 8.0 (1 ml). The suspension is stirred at 22°C for 42 hours. The reaction mixture is then centrifuged at 1000 rpm for 5 minutes, the precipitate is washed with a 0.2 M phosphate buffer at pH 8, and then centrifuged again to collect the unreacted enantiomer insoluble in water. The supernatant and aqueous phase are combined and acidified with HCl (3 N) to pH 2. The precipitate is recovered by filtration to yield the enantiomer transformed into carboxylic acid. [6]
References
[1] Chen, X., Decker, M., Higuchi, T., & Hoffmann, M. (2019). Sartan analogue. WO2019134765A1
[2] Castaldi, G., Belli, A., Uggeri, F., & Giordano, C. (1983). A Lewis acid catalyzed 1,2 aryl shift in .alpha.-haloalkyl aryl acetals: a convenient route to .alpha.-arylalkanoic acids. Journal of Organic Chemistry, 48(24), 4658–4661. https://doi.org/10.1021/jo00172a041
[3] Gao, Q., Ishihara, K., Maruyama, T., Mouri, M., & Yamamoto, H. (1994). Asymmetric hetero Diels-Alder reaction catalyzed by stable and easily prepared CAB catalysts. Tetrahedron, 50(4), 979–988. https://doi.org/10.1016/s0040-4020(01)80812-7
[4] Dallacker, F., & Schubert, J. (1975). Derivate des Methylendioxybenzols, 39. Über Polyencarbonsäureimide des Methylendioxybenzols. Chemische Berichte, 108(1), 95–108. https://doi.org/10.1002/cber.19751080113
[5] De, S. K. (2021). Applied Organic Chemistry: Reaction mechanisms and experimental procedures in medicinal chemistry. https://openlibrary.org/books/OL29476874M/Applied_Organic_Chemistry.
[6] Gu, Q., Chen, C., & Sih, C. J. (1986). A facile enzymatic resolution process for the preparation of (+)–2-(6-hethoxy-2-naphthyl)propionic acid (Naproxen). Tetrahedron Letters, 27(16), 1763–1766. https://doi.org/10.1016/s0040-4039(00)84368-3.