Introduction
Have you ever gone grocery shopping at the supermarket? Unless you’re living blissfully in the treetops of the jungle, your answer to this simple, perhaps trivial, question should be ‘YES.’ So, if you’ve been to the supermarket, you’ve definitely noticed the section dedicated to ‘personal care.’ That section that reminds you of your daily hygiene duties to live in a modern society. As bothersome as that reminder might be, it’s likely that you’ve purchased soap bars, body wash, shampoo, and other cleansers.
So, let’s get to the heart of the matter: before tossing various detergents and soaps into your cart, have you ever taken a moment to check the ingredients? Something tells me you haven’t, considering the average person’s laziness… Well! If you had, over the years you would have noticed that there is one substance that is always, always present in all these products. The substance in question is SDS, also known as SLS. In this brief article, we’ll get to know this substance and find out whether it’s dangerous to your health.
What is SDS?
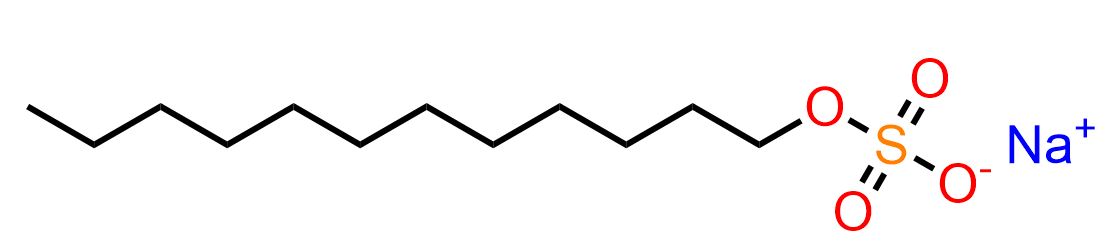
SDS stands for Sodium Dodecyl Sulfate, also known as SLS, or Sodium Lauryl Sulfate. Its chemical formula consists of 12 carbon atoms, 25 hydrogen atoms, 4 oxygen atoms, and 1 sulfur atom. The figure below illustrates the structural formula of SDS.
Being an anion, it is commercially available in the form of a sodium salt.
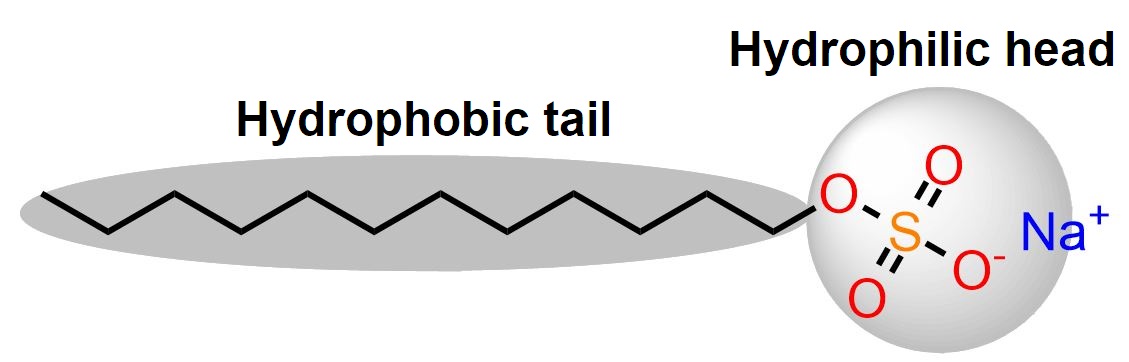
For those with a bit of a chemistry eye, the structural formula reveals that the molecule has a dual nature: a hydrophilic head (attracted to polar molecules like water) and a hydrophobic tail (attracted to non-polar substances like oil and grease) [1].
The structure of the molecule is fundamental to its function as a detergent and is the key to its success, as we will explore further.
Production
From an industrial perspective, the production of SDS occurs through the sulfonation of lauric alcohol, which reacts with sulfur trioxide (SO₃) or chlorosulfonic acid (ClSO₃H). This initial reaction is followed by a treatment with sodium hydroxide (NaOH) or sodium bicarbonate (NaHCO₃) (see figure below) [1].

Lauric alcohol is obtained from coconut oil or palm oil.
The production of sodium dodecyl sulfate is approximately 7.2 million tons per year. However, the market is expected to grow, according to the projections for the period 2024-2030 in the Global Sodium Dodecyl Sulfate (SDS) Market Research Report.
How it works?
We previously mentioned that SDS has a dual nature: a polar head and a hydrophobic tail. This structure is fundamental to its function. In the presence of water and dirt (such as greasy or oily substances), the molecule orients itself with the polar head facing the water (which is also a polar substance) and the hydrophobic tail directed towards the non-polar substance, such as grease.
At a certain concentration, SDS molecules aggregate to form spheres that trap dirt inside them (see figure below). These spheres are called micelles and are the fundamental principle behind all detergents. Micelles, by encapsulating the dirt inside, remove it from the washed surface with the help of water. In other words, SDS works by making dirt soluble in water, thereby allowing it to be easily removed.
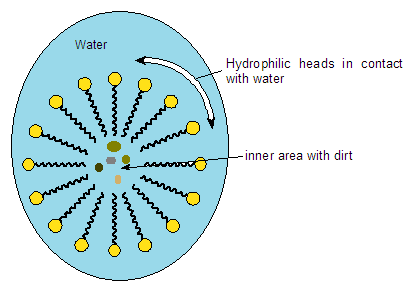
Is it harmful to health?
Let’s get to the crux of our article: is SDS, an omnipresent molecule, harmful to human health and the environment?
It is not possible to answer this question with a simple ‘yes’ or ‘no,’ as SDS has varying effects depending on the animal species, and its impact changes based on concentration and interactions with other products. Let’s address this in detail.
Enviromental impact– Given its presence in many products, SDS can easily end up contaminating water and soil. However, the good news is that SDS is highly biodegradable and is rapidly broken down by bacteria into harmless substances. It is estimated that 45-95% of SDS disappears within 24 hours [1].
Damage to Insects– For insects, SDS is far from harmless. For instance, the LC50 for housefly larvae has been determined to be 78 ppm, meaning that 50% of the larvae die when exposed to a dose of 78 ppm of pure SDS. [2]. Further studies are needed to assess the impact of SDS on other insect species.
Damage to Aquatic Life– SDS is more dangerous for invertebrates than for fish. Fish are able to expel the substance, if present as a contaminant in the water, within 24-48 hours [1].
Damage to Avian Life– The effect of SDS on birds has been studied exclusively in aquatic birds, which can come into contact with the substance through contaminated water. It has been observed that when these animals swim in water containing SDS, they can die from hypothermia or drowning. This particular effect is believed to be caused by SDS interfering with the layer of air trapped in the birds’ feathers, which helps to maintain their body temperature. SDS reduces the surface tension of water, allowing it to penetrate this layer, displace the air, and wet the bird. As a result, the animal has difficulty maintaining its body temperature, leading to a risk of death [3].
Damage to Mammals– The toxicity of SDS in mammals has been primarily studied in mice. These animals, often used in various experiments, showed a 50% mortality rate when exposed to a dose of 100-250 mg/kg of pure SDS (mg of the substance per kg of body weight) via intraperitoneal injection, while the lethal dose via intravenous administration was 118 mg/kg. [4]. Some studies have used rabbits to examine the effects of SDS on the eyes. It was observed that at a concentration of 1%, SDS does not cause significant effects. However, at a concentration of 5%, signs of temporary conjunctivitis appear, while at a concentration of 25%, substantial damage to the cornea is observed.
Damage to Humans– Humans can come into contact with SDS through skin contact during the use of household or personal care detergents, ingestion via SDS-containing toothpastes, ingestion of contaminated water, and inhalation from the release of SDS from surfaces treated with products containing this substance. To date, there have been no reported cases of fatal SDS poisoning in humans. However, it is important to consider non-lethal side effects. SDS is known to be an irritant to human skin, causing dryness, rashes, swelling, etc. The irritation depends on the duration of exposure and concentration. At concentrations below 1%, exposure for 24 hours typically does not cause significant problems. However, at higher concentrations, noticeable signs of skin irritation can appear within 24 hours. The higher the concentration of the substance, the greater the damage to the skin [5]. Additionally, SDS can cause eye irritation, as demonstrated in rabbit studies, in the event of accidental contact with the eyes due to the use of shampoos and cosmetics containing this substance.
Conclusions
Upon reviewing the last paragraph, it seems that while SDS may not be lethal, it could highly damage the skin, especially considering that it can reach concentrations of up to 50% in shampoos and body washes, far above the 1% threshold. So why, when we wash, don’t we emerge from the shower with our skin worn, eroded, and bleeding?
This does not happen because SDS becomes irritating only with prolonged exposure (over several hours). Conversely, if it is rinsed off shortly after application, it does not cause problems. Additionally, in personal care products, SDS is often combined with other substances, such as betaines, which can reduce its aggressive action.
So, is SDS dangerous? Partially yes, but it largely depends on how you use it. In any case, I wouldn’t use it as a beverage or as a face mask!
.
References
1) Singer, M. M., & Tjeerdema, R. S. (1993). Fate and effects of the surfactant sodium dodecyl sulfate. Reviews of Environmental Contamination and Toxicology, 95–149. https://doi.org/10.1007/978-1-4613-9529-4_3
2) Piper, W. D., & Maxwell, K. E. (1971). Mode of action of surfactants on Mosquito Pupae1,2. Journal of Economic Entomology, 64(3), 601–606. https://doi.org/10.1093/jee/64.3.601.
3) Russell, W. C., Choules, G. L., & Gauthier, D. A. (1981). Detergents and waterfowl. The Journal of Zoo Animal Medicine, 12(1), 10. https://doi.org/10.2307/20094499.
5) Prottey, C., & Ferguson, T.F. (1975). Factors which determine the skin irritation potential of soaps and detergents.